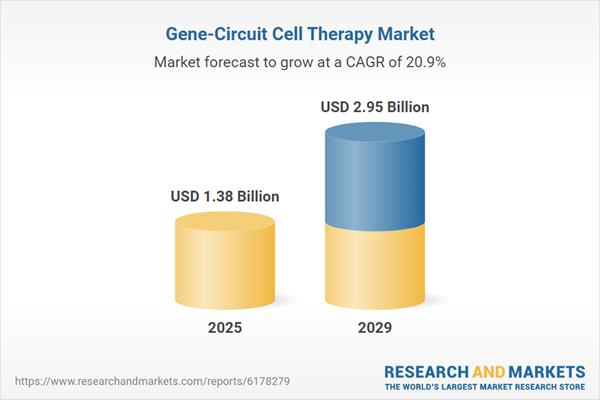
The gene-circuit cell therapy market size is expected to see exponential growth in the next few years. It will grow to $2.95 billion in 2029 at a compound annual growth rate (CAGR) of 20.9%. Growth in the forecast period is expected to result from greater adoption of in-vivo delivery and non-viral platforms, stronger regulatory support with streamlined approval processes, increasing investment from pharmaceutical companies and venture capital, rising demand for off-the-shelf allogeneic cell therapies, and the expansion of manufacturing capacity and gene synthesis services. Key trends projected for the forecast period include advancements in synthetic gene circuit design, the development of safety switches and programmable control systems, progress in automated and scalable manufacturing, and integration of technologies for real-time monitoring and bioinformatics.
The increasing incidence of genetic diseases is expected to drive the growth of the gene-circuit cell therapy market in the future. Genetic diseases are disorders caused by changes or mutations in DNA that can be inherited or arise spontaneously, impacting health, development, or bodily functions. The growing prevalence of these diseases is linked to heightened awareness and improvements in diagnostic technologies, which facilitate earlier and more accurate identification of previously undiagnosed conditions. Gene-circuit cell therapy aids in managing genetic diseases by providing highly targeted and programmable treatments, making it suitable for conditions involving complex or previously untreatable genetic defects. It improves patient outcomes by precisely modifying or controlling cellular functions, reducing disease symptoms, and potentially offering functional cures. For example, the Cystic Fibrosis Trust reported in September 2023 that 11,148 people were diagnosed with cystic fibrosis in 2022, a rise from 10,908 in 2021. This growing incidence of genetic diseases is thus contributing to the expansion of the gene-circuit cell therapy market.
The increasing use of personalized medicine is anticipated to boost the growth of the gene-circuit cell therapy market moving forward. Personalized medicine is a healthcare approach that customizes treatments and prevention strategies according to an individual’s unique genetic makeup, lifestyle, and environment. The rising adoption of personalized medicine is largely due to advancements in genomics, which allow for precise identification of genetic variations and the development of tailored treatments. Gene-circuit cell therapy supports this approach by enabling targeted and programmable treatments, making it ideal for managing complex and chronic diseases. It improves therapeutic precision by tailoring interventions to meet the specific needs of individual patients, leading to better treatment effectiveness and overall health outcomes. For instance, the Personalized Medicine Coalition (PMC) reported in February 2024 that the U.S. Food and Drug Administration (FDA) approved 26 new personalized medicines in 2023, a significant increase from 12 approvals in 2022. Therefore, the growing adoption of personalized medicine is driving the growth of the gene-circuit cell therapy market.
Key players in the gene-circuit cell therapy market are focusing on developing advanced therapeutic platforms, such as CRISPR-based gene editing systems, to enhance treatment precision, improve outcomes, and address genetic diseases that were once deemed incurable. CRISPR-based gene editing technologies enable precise modifications to the genome, allowing for the correction of genetic defects or the introduction of therapeutic genes into cells. For example, in December 2023, Vertex Pharmaceuticals Inc., a U.S.-based biopharmaceutical company, partnered with CRISPR Therapeutics Inc., a U.S.-based biotechnology company, to receive conditional marketing authorization from the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) for CASGEVY (exagamglogene autotemcel, exa-cel). This therapy is designed for patients aged 12 and older with sickle cell disease (SCD) and recurrent vaso-occlusive crises or transfusion-dependent beta thalassemia (TDT). CASGEVY works by editing hematopoietic stem cells to reactivate fetal hemoglobin production, reducing or eliminating disease symptoms and offering a potential functional cure for these conditions.
Major players in the gene-circuit cell therapy market are Tmunity Therapeutics Inc., Ginkgo Bioworks Holdings Inc., Arcellx Inc., Beam Therapeutics Inc., CRISPR Therapeutics AG, Intellia Therapeutics Inc., Orchard Therapeutics plc, Fate Therapeutics Inc., Rubius Therapeutics Inc., BlueRock Therapeutics LP (a subsidiary of Bayer AG), Precigen Inc., SparingVision, Alaunos Therapeutics Inc., Cartesian Therapeutics Inc., Lyell Immunopharma Inc., Synlogic Inc., Senti Biosciences Inc., Autolus Therapeutics plc, Cellectis S.A., and Sotio Biotech.
North America was the largest region in the gene-circuit cell therapy market in 2024. The regions covered in gene-circuit cell therapy report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the gene-circuit cell therapy market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
Gene-circuit cell therapy is an advanced approach to cell therapy in which engineered cells are designed with synthetic gene circuits that enable them to detect, process, and respond to specific biological signals in the body. This allows for controlled, targeted, and adaptable therapeutic actions such as drug release, immune modulation, or correction of dysfunctional cellular behavior, offering greater precision and safety compared to conventional cell therapies.
The main product types of gene-circuit cell therapy are autologous and allogeneic. Autologous therapy involves collecting a patient’s own cells, engineering them with synthetic gene circuits to sense and react to specific biological signals, and reinfusing them into the same patient. This therapy uses advanced technologies such as clustered regularly interspaced short palindromic repeats, transcription activator-like effector nucleases, zinc finger nucleases, and others. It is applied in the treatment of oncology, genetic disorders, infectious diseases, and additional conditions. The primary end users include hospitals and clinics, research institutions, biotechnology companies, pharmaceutical companies, and others.
The library prep system market research report is one of a series of new reports that provides library prep system market statistics, including the library prep system industry global market size, regional shares, competitors with the library prep system market share, detailed library prep system market segments, market trends, and opportunities, and any further data you may need to thrive in the library prep system industry. This library prep system market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The gene-circuit cell therapy consists of revenues earned by entities by providing services such as cell engineering and programming, patient monitoring, therapeutic response customization, and clinical trial support services. The market value includes the value of related goods sold by the service provider or included within the service offering. The gene-circuit cell therapy also includes sales of engineered therapeutic cells, synthetic gene circuit constructs, cell therapy kits, reagents, and supporting delivery systems or devices. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values and are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Gene-Circuit Cell Therapy Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on gene-circuit cell therapy market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for gene-circuit cell therapy? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The gene-circuit cell therapy market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Product Type: Autologous; Allogeneic2) By Technology: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR); Transcription Activator-Like Effector Nuclease (TALEN); Zinc Finger Nuclease (ZFN); Other Technologies
3) By Application: Oncology; Genetic Disorders; Infectious Diseases; Other Applications
4) By End-User: Hospitals And Clinics; Research Institutes; Biotechnology And Pharmaceutical Companies; Other End Users
Subsegments:
1) By Autologous: Chimeric Antigen Receptor (CAR)-T Cell Therapies; T-Cell Receptor (TCR)-T Cell Therapies; Natural Killer (NK) Cell Therapies; Stem Cell-Based Therapies2) By Allogeneic: Off-The-Shelf Chimeric Antigen Receptor (CAR)-T Cell Therapies; Off-The-Shelf Natural Killer (NK) Cell Therapies; TT-Cell Receptor (TCR)-T Cell Therapies; Stem Cell-Derived Therapies
Companies Mentioned: Tmunity Therapeutics Inc.; Ginkgo Bioworks Holdings Inc.; Arcellx Inc.; Beam Therapeutics Inc.; CRISPR Therapeutics AG; Intellia Therapeutics Inc.; Orchard Therapeutics plc; Fate Therapeutics Inc.; Rubius Therapeutics Inc.; BlueRock Therapeutics LP (a subsidiary of Bayer AG); Precigen Inc.; SparingVision; Alaunos Therapeutics Inc.; Cartesian Therapeutics Inc.; Lyell Immunopharma Inc.; Synlogic Inc.; Senti Biosciences Inc.; Autolus Therapeutics plc; Cellectis S.A.; Sotio Biotech
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Gene-Circuit Cell Therapy market report include:- Tmunity Therapeutics Inc.
- Ginkgo Bioworks Holdings Inc.
- Arcellx Inc.
- Beam Therapeutics Inc.
- CRISPR Therapeutics AG
- Intellia Therapeutics Inc.
- Orchard Therapeutics plc
- Fate Therapeutics Inc.
- Rubius Therapeutics Inc.
- BlueRock Therapeutics LP (a subsidiary of Bayer AG)
- Precigen Inc.
- SparingVision
- Alaunos Therapeutics Inc.
- Cartesian Therapeutics Inc.
- Lyell Immunopharma Inc.
- Synlogic Inc.
- Senti Biosciences Inc.
- Autolus Therapeutics plc
- Cellectis S.A.
- Sotio Biotech
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.38 Billion |
| Forecasted Market Value ( USD | $ 2.95 Billion |
| Compound Annual Growth Rate | 20.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |