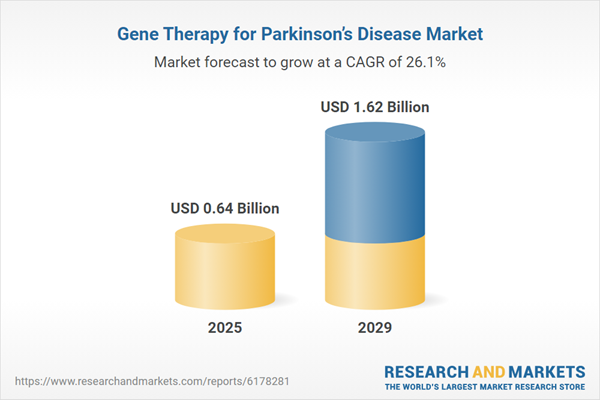
The gene therapy for parkinson’s disease market size is expected to see exponential growth in the next few years. It will grow to $1.62 billion in 2029 at a compound annual growth rate (CAGR) of 26.1%. The growth in the forecast period is driven by greater adoption of viral vector-based therapies, increased investment in gene therapy startups, rising government support for rare disease treatments, expansion of research collaborations, and development of healthcare infrastructure in emerging markets. Key trends in the forecast period include technological advancements in viral delivery systems, innovations in neurotrophic factor therapies, development of targeted gene-editing constructs, increased research and development investment, and progress in precision medicine approaches.
The increasing interest in personalized medicine is expected to drive the growth of the gene therapy for Parkinson’s disease market. Personalized medicine is a healthcare approach that customizes treatment and prevention strategies based on an individual’s genetic profile, lifestyle, and environmental factors. Interest in this approach continues to rise due to growing demand for targeted therapies that enhance treatment effectiveness and reduce unwanted side effects. In the context of Parkinson’s disease, gene therapy supports personalized medicine by delivering tailored genetic modifications that aim to restore dopamine function and address unique disease mechanisms specific to each patient. For example, in February 2024, the Personalized Medicine Coalition, a nonprofit organization based in the United States, reported that in 2023, the United States Food and Drug Administration approved sixteen new personalized treatments for rare disease patients, up from six approvals in 2022. As a result, the growing interest in personalized medicine is contributing to the expansion of the gene therapy for Parkinson’s disease market.
Companies operating in the gene therapy for Parkinson’s disease market are actively developing more precise treatments such as regenerative medicine advanced therapies to promote neuron survival, restore dopamine production, and provide durable disease-modifying benefits for individuals with Parkinson’s disease. Regenerative medicine advanced therapy is a designation granted by the United States Food and Drug Administration to regenerative medicine products, including cell and gene therapies as well as tissue-engineered products, that aim to treat, reverse, or cure serious or life-threatening conditions. For example, in February 2025, Asklepios BioPharmaceutical Inc., a gene therapy company based in the United States, introduced AB-1005, an experimental gene therapy using adeno-associated virus to deliver glial cell line-derived neurotrophic factor. This therapy received regenerative medicine advanced therapy designation from the FDA. The designation was based on clinical data from a Phase Ib open-label, uncontrolled trial, which demonstrated AB-1005’s potential to slow the progression of Parkinson’s disease and improve motor function.
In January 2025, BlackfinBio Limited, a company based in the United Kingdom that specializes in clinical-stage gene therapy for rare neurological and dopamine-related disorders, acquired the Parkinson’s disease patent portfolio from Oxford Biomedica plc for an undisclosed amount. With this acquisition, BlackfinBio aims to further develop its BFB-201 program for Parkinson’s disease and benefit from Oxford Biomedica’s intellectual property in gene and cell therapy to accelerate therapeutic innovation. Oxford Biomedica plc is a company based in the United Kingdom that develops gene and cell therapies and provides vector technologies, offering valuable expertise and intellectual assets.
Major players in the gene therapy for parkinson’s disease market are Merck & Co. Inc., Bayer AG, Sanofi S.A., Eli Lilly and Company, Biogen Inc., PTC Therapeutics Inc., Zambon S.p.A., Oxford BioMedica plc, Voyager Therapeutics Inc., Insilico Medicine Inc., Spur Therapeutics Inc., Cerevance Inc., MeiraGTx Holdings plc, BlackfinBio Inc., Partner Therapeutics Inc., Kenai Therapeutics Inc., CureSen Therapeutics Inc., BioVie Inc., Clexio Biosciences Ltd., Tetraneuron S.L.
North America was the largest region in the gene therapy for parkinson’s disease market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in gene therapy for parkinson’s disease report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the gene therapy for parkinson’s disease market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
Gene therapy for Parkinson’s disease involves using genetic techniques to introduce, repair, or regulate genes within brain cells. The therapy aims to restore dopamine production, protect nerve cells, or correct disease-related mutations, with the goal of slowing disease progression and providing long-term symptom relief.
The primary therapy types of gene therapy for Parkinson’s disease are in vivo gene therapy and ex vivo gene therapy. In vivo gene therapy involves the direct delivery of genetic material into the patient’s body, typically using viral or non-viral vectors, to restore or enhance the function of specific genes associated with Parkinson’s disease. Target genes include aromatic L-amino acid decarboxylase (AADC), glial cell line-derived neurotrophic factor (GDNF), neurturin, and other related genes. These therapies are administered via intracerebral, intravenous, and other delivery methods. Key end-users include hospitals, specialty clinics, research institutes, and related healthcare facilities.
The gene therapy for parkinson’s disease market research report is one of a series of new reports that provides gene therapy for parkinson’s disease market statistics, including the gene therapy for parkinson’s disease industry global market size, regional shares, competitors with the gene therapy for parkinson’s disease market share, detailed gene therapy for parkinson’s disease market segments, market trends, and opportunities, and any further data you may need to thrive in the gene therapy for parkinson’s disease industry. This gene therapy for parkinson’s disease market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The gene therapy for parkinson’s disease market consists of revenues earned by entities by providing services such as genetic counseling, preclinical studies, clinical trial management, and personalized treatment planning. The market value includes the value of related goods sold by the service provider or included within the service offering. The gene therapy for parkinson’s disease market also includes sales of viral vectors, aromatic L-amino acid decarboxylase (AADC) gene therapy, glutamic acid decarboxylase (GAD) gene therapy, glial cell line-derived neurotrophic factor (GDNF) or neurturin-based neurotrophic therapies, and glucocerebrosidase (GBA)-targeted gene therapies. Values in this market are ‘factory gate’ values; that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values and are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Gene Therapy For Parkinson’s Disease Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on gene therapy for parkinson’s disease market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for gene therapy for parkinson’s disease? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The gene therapy for parkinson’s disease market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Therapy Type: In Vivo Gene Therapy; Ex Vivo Gene Therapy2) By Vector Type: Viral Vectors; Non-Viral Vectors
3) By Target Gene: Aromatic L-Amino Acid Decarboxylase (AADC); Glial Cell Line-Derived Neurotrophic Factor (GDNF); Neurturin; Other Target Genes
4) By Delivery Method: Intracerebral; Intravenous; Other Delivery Methods
5) By End-User: Hospitals; Specialty Clinics; Research Institutes; Other End-Users
Subsegments:
1) By In Vivo Gene Therapy: Viral Vectors; Non-Viral Vectors2) By Ex Vivo Gene Therapy: Autologous Cell-Based Gene Therapy; Allogeneic Cell-Based Gene Therapy
Companies Mentioned: Merck & Co. Inc.; Bayer AG; Sanofi S.A.; Eli Lilly and Company; Biogen Inc.; PTC Therapeutics Inc.; Zambon S.p.A.; Oxford BioMedica plc; Voyager Therapeutics Inc.; Insilico Medicine Inc.; Spur Therapeutics Inc.; Cerevance Inc.; MeiraGTx Holdings plc; BlackfinBio Inc.; Partner Therapeutics Inc.; Kenai Therapeutics Inc.; CureSen Therapeutics Inc.; BioVie Inc.; Clexio Biosciences Ltd.; Tetraneuron S.L.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Gene Therapy for Parkinson’s Disease market report include:- Merck & Co. Inc.
- Bayer AG
- Sanofi S.A.
- Eli Lilly and Company
- Biogen Inc.
- PTC Therapeutics Inc.
- Zambon S.p.A.
- Oxford BioMedica plc
- Voyager Therapeutics Inc.
- Insilico Medicine Inc.
- Spur Therapeutics Inc.
- Cerevance Inc.
- MeiraGTx Holdings plc
- BlackfinBio Inc.
- Partner Therapeutics Inc.
- Kenai Therapeutics Inc.
- CureSen Therapeutics Inc.
- BioVie Inc.
- Clexio Biosciences Ltd.
- Tetraneuron S.L.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 0.64 Billion |
| Forecasted Market Value ( USD | $ 1.62 Billion |
| Compound Annual Growth Rate | 26.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |