The vaccine development companies produced an estimated 7 billion doses of vaccines globally, in the year 2024 and are expected to produce around 8 billion doses in 2025.
Vaccine Developers Market: Growth and Trends
Vaccine is a specialized medical product formulated to activate the body’s immune system, enabling it to recognize and protect against specific diseases over the long term. The development of vaccines typically involves using either weakened or inactivated forms of the disease-causing microorganism, or including crucial antigenic components from the pathogen, such as structural proteins and inactivated toxins. By introducing these components, vaccines assist the immune system in recognizing and combating the real pathogen if encountered in the future.
According to World Health Organization, vaccines are categorized into two types, namely preventive (prophylactic) vaccines, which are administered to protect individuals from contracting diseases, and therapeutic vaccines, which are utilized as treatment regimens to help the body fight existing illnesses. Currently, pharmaceutical companies offer numerous vaccines, and many are undergoing clinical trials or are in various stages of development, targeting a wide range of serious and debilitating conditions.
Recently, the Coalition for Epidemic Preparedness Innovations (CEPI) has made a significant new investment of USD 24 million in the Vaccine and Infectious Disease Organization (VIDO), aiming to bolster global preparedness for future infectious disease outbreaks as the world continues to combat the last pandemic.
Significantly, the development and approval of innovative vaccines, by vaccine development companies, such as mRNA, DNA, and recombinant vaccines, are significant trends influencing the competitive landscape of vaccines market.
Vaccine Developers Market: Key Insights
The report delves into the current state of the vaccine developers market and identifies potential growth opportunities within industry.
Some key findings from the report include:
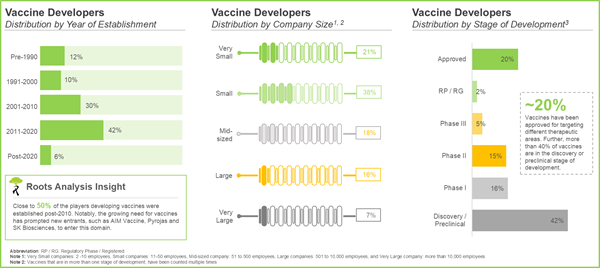
- The current market landscape is highly fragmented, featuring the presence of both new entrants and established players; of these, close to 25% of the vaccine developers are large and very large players.
- 35% vaccines are being evaluated across different phases of clinical trials; notably, more than 40% of the vaccines are under discovery / preclinical stage.
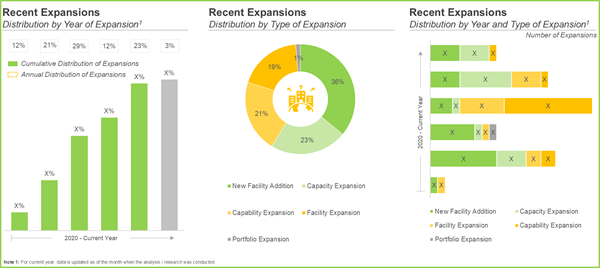
- Most of the expansion initiatives were undertaken in the past few years; of these, nearly 55% of the instances were focused on enhancing the existing vaccine development capabilities to meet increasing demand.
- Majority of the recent expansions (~40%) were undertaken in facilities located in Asia-Pacific; notably, India and Singapore emerged as the key regions where most of the expansions were undertaken.
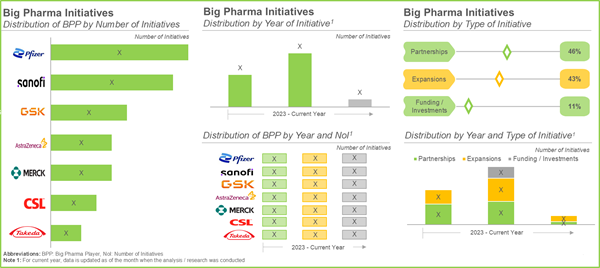
- Close to 50% of the vaccines-related partnerships were inked in the year 2024; of these, more than 15% of the agreements were manufacturing and product development agreements.
- Over 45% of the big pharma players have signed various partnerships to enhance their existing vaccine portfolios, followed by players (43%) undertaking several expansion initiatives.
Vaccine Developers Market: Key Segments
Competitive Landscape of Vaccine Development Companies
The current market landscape takes into account more than 200 vaccine developers including very large, large, mid-sized and small companies. These vaccine developers have the required expertise to develop different types of vaccines, such as cancer vaccines, mRNA vaccines, RSV vaccines and DNA vaccine across different key geographical regions. Notably, most companies are based in North America, followed by Asia-Pacific; this is reflective of the high market potential in these regions. Notable examples of recently established vaccine developers (established post-2020, in alphabetical order) include AbVacc, Auro Vaccines, HilleVax, Innovac Therapeutics, Nuravax, Pyrojas and Vernagen.
Rising Partnerships and Collaborations Activity to Foster Progress and Innovation in Expanding their Capabilities
The vaccine development market has witnessed a decent partnership activity in the past few years. Notably, in the last five years, companies based in North America and Europe have inked majority of the partnerships to strengthen their development processes of vaccines. Most of the partnerships signed in this domain are manufacturing agreements, followed by product development agreements. This is indicative of the persistent efforts of vaccine developers to advance their research and development activities for the development of different types of vaccines.
Recent Facility Expansions to Improve Capacity
The vaccine development players have witnessed significant facility expansions to meet growing global demand and advance manufacturing capabilities. In March 2025, Merck opened a USD 1 billion state-of-the-art vaccine manufacturing plant in North Carolina, incorporating cutting-edge technologies, such as AI and 3D printing. Other key players, including Lonza and WuXi Biologics, have also expanded their new mRNA vaccines in development and viral vector vaccine capacities worldwide. These investments emphasize the sector's commitment to increasing production, boosting innovation, and reinforcing supply chains to fulfill future vaccine needs.
Vaccine Development Companies Landscape: Research Coverage
- Market Landscape: An in-depth assessment of the companies involved in the vaccine development market, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] purpose of vaccine, [E] type of vaccine [F] type of subunit vaccine, [G] therapeutic area, and [H] type of infectious diseases targeted.
- Recent Expansions: A detailed analysis of the expansion initiatives undertaken in the vaccine development domain, on the basis of [A] year of expansion, [B] type of expansion, [C] location of expanded facility, [D] area of expanded facility, [E] amount invested and [F] purpose of expansion
- Partnerships and Collaborations: An in-depth analysis of the partnership’s activity reported in this domain, based on parameters such as [A] year of partnership, [B] type of partnership [C] type of partner, [D] type of vaccine, [D] therapeutic area and [E] geographical distribution.
- Big Pharma Initiatives: A detailed analysis of big pharma initiatives reported in the domain, on the basis of [A] year of initiative, [B] type of partnership, [C] type of expansion, and [D] type of vaccine focused.
- Cost Price Analysis: An in-depth cost price analysis depicting [A] key historical trends in vaccine production costs, [B] vaccine cost structure, [C] factors influencing vaccine prices, [D] investment considerations associated with vaccines production and [E] cost-saving strategies in vaccine development and manufacturing processes.
Sample Players in the Vaccine Development Companies Landscape, Profiled in the Report Include:
- AstraZeneca
- CSL
- GlaxoSmithKline
- Merck
- Pfizer
- Sanofi
- Takeda
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AstraZeneca
- CSL
- GlaxoSmithKline
- Merck
- Pfizer
- Sanofi
- Takeda
Methodology

LOADING...