United States Artificial Insemination Industry Overview
The intentional implantation of sperm into a woman's reproductive system in order to induce pregnancy without sexual contact is known as artificial insemination, or AI. It is frequently used for same-sex partners hoping to conceive, infertile couples, and single women. Depending on clinical needs, the procedure can be carried out intrauterinely (IUI), intracervically (ICI), or intratubally (ITI) using the partner's or donor sperm. To boost success rates, AI is frequently used in conjunction with hormonal stimulation or ovulation tracking. AI has become a commonly used, minimally invasive assisted reproductive technology as a result of improvements in sperm preparation, storage, and timing..The market for artificial insemination in the United States is growing as a result of greater awareness of assisted reproductive technologies (ART) and rising incidence of infertility among couples. Medical issues, lifestyle choices, and delayed motherhood all increase the need for fertility treatments. Adoption has increased due to greater success rates brought about by technological developments in cryopreservation, handling sperm and embryos, and monitoring methods. Accessible treatment choices are offered by fertility clinics and specialty medical facilities, and financial obstacles are lessened by reimbursement schemes and insurance policies that are supportive. The need for artificial insemination treatments is also being driven nationwide by the increasing acceptance of ART among a variety of demographics, such as same-sex couples and single parents.
Growth Drivers for the United States Artificial Insemination Market
Rising Infertility Rates
One major factor propelling the artificial insemination (AI) market is the rising incidence of infertility in the US. The general fertility rate in 2023 was 54.5 births per 1,000 women aged 15-44, down from 56 in 2022, according to the Centers for Disease Control and Prevention (CDC). This suggests that fertility rates are continuing to drop. Numerous variables, such as delayed parenthood, lifestyle decisions, and fertility-affecting medical issues, are blamed for this trend. In order to become pregnant, more people and couples are turning to assisted reproductive technologies like artificial intelligence. Improvements in reproductive medicine, greater understanding, and easier access to fertility treatments all contribute to the rising demand for AI services. It is anticipated that this confluence of elements will support the ongoing expansion of the AI sector in the United States.Awareness of Assisted Reproductive Technologies (ART)
The market for artificial insemination in the United States is mostly driven by rising knowledge of assisted reproductive technologies (ART). Intrauterine insemination (IUI) and in vitro fertilization (IVF) are two treatment options that couples, single parents, and LGBTQ+ people are now more aware of thanks to increased education and media coverage on infertility remedies. The success stories, advantages, and safety of ART are highlighted in public debates, social media campaigns, and community health initiatives, which helps to dispel myths and social stigma. In order to overcome reproductive difficulties, more people are actively seeking fertility services. Additionally, patients are receiving education from healthcare experts regarding enhanced reproductive procedures, individualized treatment programs, and early treatments. Growing understanding and improvements in reproductive medicine lead to increased use of artificial insemination treatments, which boosts the industry as a whole and makes fertility services available to a wider range of Americans.Accessible Fertility Clinics
The market for artificial insemination is growing as a result of the opening of more fertility clinics and specialized reproductive healthcare facilities across the US. Fertility services are now more widely available, making them more accessible to urban, suburban, and increasingly rural people. Patients can obtain individualized care in convenient settings thanks to modern clinics' state-of-the-art facilities, skilled doctors, and extensive fertility programs. Furthermore, the overall experience and success rates of artificial insemination operations are improved by the incorporation of cutting-edge technologies, patient counseling, and support services. Additionally, accessibility eases patients' travel and logistical burdens, increasing the number of people who can afford fertility treatments. Because of this, more people and couples are inspired to seek assisted reproductive technologies, which increases the uptake of artificial insemination and supports the market's steady expansion in the United States.Challenges in the United States Artificial Insemination Market
High Costs
In the US market for artificial insemination, high treatment costs continue to be a major obstacle. Intrauterine insemination (IUI) and related fertility treatments are costly procedures that frequently call for several cycles, certain drugs, and diagnostic testing. Patients must pay for assisted reproductive technologies out of pocket because many insurance plans cover them only partially or not at all. Couples and individuals may be deterred from pursuing therapy or finishing the several cycles required for a healthy pregnancy by this financial strain. Even with partial coverage, the total cost is increased by related expenses including travel, consultation fees, and monitoring. In order to make artificial insemination available to a larger population, encourage market growth, and guarantee that more people can take use of fertility services, it is imperative to lower costs, increase insurance reimbursement, and provide financing options.Regulatory and Ethical Considerations
One of the biggest obstacles facing the US artificial insemination sector is ethical and regulatory issues. Complex federal and state laws controlling donor screening, processing of sperm and embryos, consent processes, and reporting obligations apply to fertility treatments. Adherence to these regulations raises operating expenses and could impede the adoption of novel technologies or creative processes. Public opinion and acceptability may also be impacted by ethical issues pertaining to genetic screening, donor selection, embryo preservation, and the rights of all parties. Clinics have to navigate regulatory frameworks while upholding high ethical standards, which can be administratively taxing. To maintain patient safety, legal compliance, and public confidence while promoting the development and accessibility of assisted reproductive technologies, these issues need to be carefully managed.California Artificial Insemination Market
California is a leading market for artificial insemination due to its large, diverse population and advanced healthcare infrastructure. Urban centers like Los Angeles and San Francisco have numerous specialized fertility clinics offering assisted reproductive technologies, including IUI and IVF. High awareness of fertility options, lifestyle factors, and delayed parenthood contribute to growing demand. The state also sees strong adoption among single parents and LGBTQ+ couples seeking reproductive assistance. Availability of advanced reproductive technologies, expert specialists, and supportive counseling services enhances patient confidence and success rates. Additionally, public and private initiatives promoting reproductive health education and access to fertility services further drive adoption. California’s combination of medical expertise, population diversity, and progressive health policies positions it as a major contributor to the U.S. artificial insemination market.Texas Artificial Insemination Market
Texas represents a rapidly growing market for artificial insemination, shaped by its large population and increasing awareness of fertility treatments. Urban centers like Houston, Dallas, and Austin host advanced fertility clinics offering comprehensive assisted reproductive services, including intrauterine insemination. Delayed parenthood, lifestyle-related infertility, and rising acceptance among single parents and LGBTQ+ individuals contribute to demand. Clinics are expanding to provide patient counseling, monitoring, and innovative treatment options, improving convenience and success rates. In rural areas, mobile fertility programs and telehealth consultations are gradually improving access. Growing awareness campaigns and community outreach initiatives help reduce social stigma and encourage adoption. Combined with technological advancements, these factors make Texas a key growth market in the U.S. artificial insemination sector.New York Artificial Insemination Market
New York, particularly New York City, is a significant market for artificial insemination due to high population density and strong healthcare infrastructure. Fertility clinics in urban areas offer advanced reproductive technologies, including intrauterine insemination and IVF, supported by specialized medical staff. Delayed parenthood, career-focused lifestyles, and rising infertility awareness drive demand. Public education campaigns and social acceptance of assisted reproduction among diverse populations, including single parents and same-sex couples, encourage treatment adoption. Urban accessibility, telemedicine consultations, and patient support services enhance convenience and treatment success. The combination of medical expertise, patient awareness, and technological advancement strengthens New York’s position as a leading market for artificial insemination in the United States.Florida Artificial Insemination Market
Florida’s artificial insemination market is shaped by its diverse population, strong healthcare network, and increasing awareness of fertility solutions. Urban centers, such as Miami and Orlando, provide advanced fertility clinics offering intrauterine insemination, IVF, and related reproductive technologies. Growing demand is driven by delayed parenthood, lifestyle factors, and acceptance of assisted reproduction among single parents and LGBTQ+ couples. Clinics focus on comprehensive patient care, including counseling, monitoring, and personalized treatment plans, to enhance convenience and success rates. Telehealth and outreach programs are gradually improving access in suburban and rural areas. A combination of medical expertise, technological innovation, and public awareness initiatives positions Florida as an important contributor to the U.S. artificial insemination market.Recent Developments in United States Artificial Insemination Market
- In October 2024, Femasys partnered with Boston IVF to offer FemaSeed, an innovative artificial insemination solution, across nearly 30 U.S. centers. This collaboration enhances market growth by expanding infertility treatment access through advanced technologies like freezing sperm, increasing awareness and social acceptance.
- In March 2024, Femasys Inc. announced positive topline results from a pivotal trial for FemaSeed, an innovative artificial insemination device. The trial reported a 24% pregnancy rate in women with severe male factor infertility, significantly surpassing conventional IUI rates, supporting market growth and ongoing commercialization.
- In December 2023, the FDA granted Mosie Baby clearance for the first over-the-counter (OTC) at-home artificial insemination kit, enhancing market growth by increasing access to fertility care. The kit supports both fresh and frozen sperm and offers a comfortable, user-friendly design.
United States Artificial Insemination Market Segments:
Type
- Intrauterine
- Intracervical
- Intravaginal
- Intratubal
Source Type
- AIH-Husband
- AID-Donor
End Use
- Hospitals and Clinics
- Fertility Centers
- Other End Users
States - Market breakup in 29 viewpoints:
- California
- Texas
- New York
- Florida
- Illinois
- Pennsylvania
- Ohio
- Georgia
- New Jersey
- Washington
- North Carolina
- Massachusetts
- Virginia
- Michigan
- Maryland
- Colorado
- Tennessee
- Indiana
- Arizona
- Minnesota
- Wisconsin
- Missouri
- Connecticut
- South Carolina
- Oregon
- Louisiana
- Alabama
- Kentucky
- Rest of United States
All companies have been covered from 5 viewpoints:
- Company Overview
- Key Persons
- Recent Development & Strategies
- SWOT Analysis
- Sales Analysis
Key Players Analysis
- Vitrolife
- Genea Pty Limited
- Rinovum Women’s Health, LLC
- Pride Angel
- HI-TECH SOLUTIONS
- FUJIFILM Irvine Scientific
- Kitazato Corporation
- Rocket Medical plc
- Conceivex, Inc.
Table of Contents
Companies Mentioned
- Vitrolife
- Genea Pty Limited
- Rinovum Women’s Health, LLC
- Pride Angel
- HI-TECH SOLUTIONS
- FUJIFILM Irvine Scientific
- Kitazato Corporation
- Rocket Medical plc
- Conceivex, Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2025 |
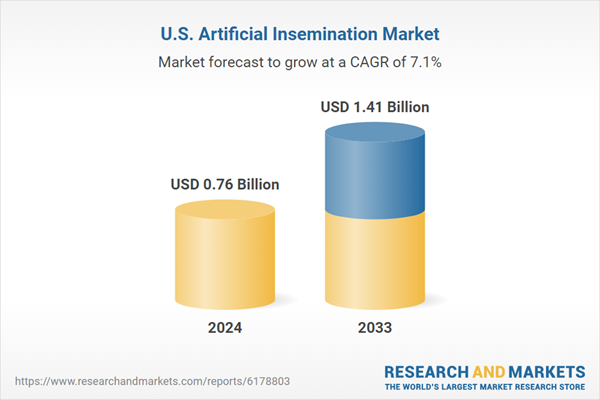
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 0.76 Billion |
| Forecasted Market Value ( USD | $ 1.41 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | United States |
| No. of Companies Mentioned | 9 |