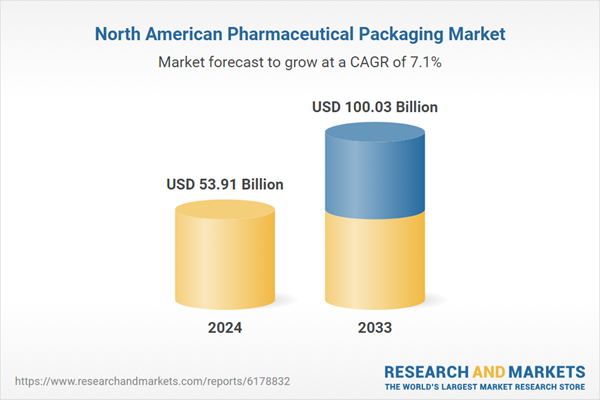
North America Pharmaceutical Packaging Industry Overview
Pharmaceutical packaging encompasses the materials, containers, and technologies used to protect, preserve, and safely distribute pharmaceuticals to patients. It covers both main packaging (bottles, vials, blister packs, and syringes) and secondary packaging (cartons and labels). The packaging method assures medicine stability, prevents contamination, and preserves efficacy throughout the supply chain. To protect patient safety, regulatory bodies such as the FDA impose stringent labeling, serialization, and tamper-evidence requirements. Advanced package technologies, including as smart packaging and anti-counterfeit solutions, improve patient compliance and safety, making pharmaceutical packaging an essential component of the healthcare ecosystem.The North American pharmaceutical packaging industry is primarily driven by increased pharmaceutical manufacturing, notably for biologics, specialty, and personalized medications. Regulatory regulations from organizations such as the FDA prioritize patient safety, labeling accuracy, and tamper-evident packaging, increasing demand for innovative solutions. The growth of e-pharmacies and online medicine distribution necessitates secure, long-lasting, and protective packaging. Smart packaging, blister packs, and sustainable materials are all examples of technological improvements that improve efficiency and safety. Furthermore, increased awareness of counterfeit pharmaceuticals has spurred the introduction of anti-counterfeit features such as serialization and tamper-evident designs. These factors combine to boost the North American pharmaceutical packaging industry, encouraging innovation and higher usage throughout the healthcare and pharmaceutical sectors.
Growth Drivers for the North America Pharmaceutical Packaging Market
Rising Pharmaceutical Production
Rising pharmaceutical output is a significant driver of the North American pharmaceutical packaging market. Leading pharmaceutical manufacturers in the United States and Canada produce an increasing volume of generic medications, biologics, vaccines, and specialty medicines. Increased production need high-quality packaging to assure product stability, integrity, and safety along the whole supply chain. The COVID-19 pandemic demonstrated the need of packaging in vaccine distribution, emphasizing temperature-controlled solutions, tamper-evident seals, and secure containers. The FDA and other regulatory bodies need tight labeling, serialization, and safety standards, which drives demand even higher. As pharmaceutical manufacturing expands to satisfy healthcare demands, the demand for innovative, compliant packaging solutions rises, facilitating market growth.Technological Advancements
Technological breakthroughs are altering the North American pharmaceutical packaging industry. Smart packaging, which includes RFID tags, QR codes, and sensors, enables real-time temperature, humidity, and tamper evidence monitoring, hence improving drug safety and traceability. Blister packs, pre-filled syringes, and modular packaging enhance dose accuracy, patient compliance, and operational efficiency. Environmental concerns and legislative incentives are driving demand for sustainable and eco-friendly materials. Major pharmaceutical corporations are investing in automation, serialization, and anti-counterfeit technologies to meet FDA and Health Canada criteria while maintaining secure supply chains. These technical advancements stimulate adoption among manufacturers, e-pharmacies, and distributors, resulting in increased market growth and modernized packaging processes.Growth of E-Pharmacies and Online Distribution
The growth of e-pharmacies and online drug distribution is fueling demand for pharmaceutical packaging in North America. As more patients purchase medications online, packaging must ensure product protection during shipping, prevent tampering, and maintain compliance with safety regulations. Durable containers, tamper-evident seals, and secure cartons are increasingly essential for maintaining drug efficacy and patient safety. Additionally, subscription-based and direct-to-consumer delivery models require packaging designed for multiple doses, clear labeling, and user convenience. The trend toward online pharmacy platforms, accelerated by the COVID-19 pandemic, has created sustained demand for innovative, secure, and sustainable packaging solutions. This shift is transforming how pharmaceutical products are packaged, stored, and delivered.Challenges in the North America Pharmaceutical Packaging Market
Regulatory Compliance
Regulatory compliance is a significant concern in the North American pharmaceutical packaging sector. To protect patient safety and combat counterfeiting, agencies like as the United States Food and Drug Administration and Health Canada impose strict labeling, tamper-evidence, serialization, and traceability regulations. Meeting these standards necessitates the purchase of advanced equipment, quality assurance procedures, and employee training. Small and medium-sized firms may have difficulty adhering to new laws, resulting in delays or penalties. Constant revisions to regulatory frameworks, particularly environmental and sustainability criteria, increase complexity. Maintaining compliance is critical but expensive and time-consuming, posing a substantial operational issue for pharmaceutical packaging companies.High Implementation and Maintenance Costs
High implementation and maintenance costs present a significant obstacle to North America's pharmaceutical packaging sector. Smart packaging, tamper-evident systems, RFID tagging, and automated production lines all need a significant capital investment. Furthermore, continual maintenance, quality control, and employee training all contribute to operational costs. Smaller manufacturers and startups frequently struggle to absorb these costs, limiting their capacity to compete with larger companies. High costs might also have an impact on pricing tactics, reducing product affordability. Despite the advantages of sophisticated and compliant packaging, the cost expense of adoption remains a significant obstacle to industry growth.United States Pharmaceutical Packaging Market
The U.S. pharmaceutical packaging market is the largest in North America, driven by high pharmaceutical production, advanced healthcare infrastructure, and stringent FDA regulations. Demand is fueled by biologics, specialty drugs, vaccines, and a growing e-pharmacy sector requiring secure, tamper-evident, and temperature-controlled packaging. Technological advancements such as smart packaging, serialization, and anti-counterfeit solutions enhance safety and compliance. Challenges include regulatory complexity, high implementation costs, and supply chain disruptions. Market growth is supported by rising healthcare expenditure, focus on patient safety, and innovation in sustainable packaging materials. Major companies like Amcor, WestRock, and Gerresheimer dominate the market.Canada Pharmaceutical Packaging Market
Canada’s pharmaceutical packaging market is expanding steadily, supported by growing pharmaceutical manufacturing and healthcare services. Regulatory compliance with Health Canada standards, including labeling, serialization, and tamper-evident packaging, drives adoption of advanced solutions. The rise of e-pharmacies and specialty drugs increases demand for secure, durable, and patient-friendly packaging. Technological innovations such as smart packaging and eco-friendly materials are gaining traction to improve safety and sustainability. Key challenges include supply chain limitations, high operational costs, and strict compliance requirements. Despite these hurdles, Canada’s market growth is fueled by rising healthcare expenditure, focus on patient adherence, and increasing demand for innovative, safe, and reliable packaging solutions.Recent Developments in North America Pharmaceutical Packaging Market
- In January 2025, DS Smith launched an innovative temperature-controlled packaging solution for the pharmaceutical industry. The solution is designed to support the sustainability targets of pharmaceutical and biotech businesses, meeting their need to store and transport delicate medicinal products across multiple territories within rigorously controlled temperature environments
- In October 2024, Bayer launched a first-of-its-kind in the healthcare industry, polyethylene terephthalate (PET) blister packaging on its renowned brand, Aleve. Realized in partnership with pharma packaging specialist Liveo Research, this innovative solution reduces the carbon footprint of this packaging by 38% and marks a stride in environmental stewardship by eliminating the use of polyvinyl chloride (PVC).
- In September 2024, BGS Beta-Gamma-Service, a specialist for more than 40 years in the use of beta and gamma rays for radiation sterilisation for medical devices, pharmaceutical packaging, and biotechnology products, announced its plan to open its first facility in the United States next year. The new 100,000-square-foot plant will offer fully automated E-Beam sterilisation and is in Imperial, PA, near the Pittsburgh International Airport. The facility is expected to be operational in mid-2025 and will operate as BGS US with Leonard Zuba, formerly vice president of sales at Raumedic, as its general manager.
- January 2024 - SGD Pharma launched the extension of its Clareo range to include 10ml and its Sterinity range of ready-to-use vials in sizes 10ml and 20m. The Clareo range of molded glass vials is available in sizes from 10ml to 200ml. These vials are designed to meet American market specifications and have a GPI 20 neck finish.
North America Pharmaceutical Packaging Market Segments:
Material
- Plastics & Polymers
- Paper & Paperboard
- Glass
- Aluminum Foil
- Others
Product
- Bottles
- Caps & Closures
- Pre-Filled Syringes
- Vials & Ampoules
- Jars & Canisters
- Blister Packs
- Pre-Filled Inhalers
- Cartridges
- Bags & Pouches
- Others
Country
- United States
- Canada
All companies have been covered from 5 viewpoints:
- Company Overview
- Key Persons
- Recent Development & Strategies
- SWOT Analysis
- Sales Analysis
Key Players Analysis
- Amcor PLC
- 3M Company
- Schott AG
- WestRock Company
- Berry Global Group Inc.
- McKesson Corporation
- AptarGroup Inc.
- Klockner Pentaplast Group
- CCL Industries Inc.
- FlexiTuff International Ltd
Table of Contents
Companies Mentioned
- Amcor PLC
- 3M Company
- Schott AG
- WestRock Company
- Berry Global Group Inc.
- McKesson Corporation
- AptarGroup Inc.
- Klockner Pentaplast Group
- CCL Industries Inc.
- FlexiTuff International Ltd
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 53.91 Billion |
| Forecasted Market Value ( USD | $ 100.03 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | North America |
| No. of Companies Mentioned | 10 |