North America 3D Printing in Healthcare Industry Overview
As technological breakthroughs change the medical scene, the North American 3D printing market for healthcare is expanding at a revolutionary rate. 3D printing is being used more and more by healthcare providers to create surgical models, implants, prosthetics, and dental treatments tailored to each patient. The technology is very useful for surgical planning and cutting down on treatment times because it facilitates efficient, personalized, and reasonably priced healthcare solutions. Additionally, the region's medical industry now has more room to grow thanks to advancements in biocompatible materials and the growing need for individualized care. Adoption is also being accelerated by collaborations between technology companies, research groups, and healthcare institutions. Market expansion is further supported by regulatory approvals for 3D-printed medical products, especially in the fields of dentistry and orthopedics. In order to improve patient care and results, hospitals and clinics are using 3D printing to create precise anatomical models for preoperative simulations and education. Additionally, the ability to produce sophisticated devices at lower costs has led to a wider adoption of this technology.When a government grants a monopoly for a certain process for a finite amount of time, patents are crucial. Other manufacturers might not be able to fully utilize the technology's advantages at that time as the patent owner has the sole right to use it. Over 30 years have passed since the invention of 3D printing, and some patents have just expired. The current patent term is determined by the United States Patent and Trademark Office (USPTO) at 20 years from the date of the patent application. It is projected that the growing advantages of patent expiration, such as less expensive development and manufacturing in the healthcare sector, will propel market expansion. For example, May 2022 research in the Survey of Ophthalmology found that 3D printing is the subject of more than 12,000 patents. Many of them are related to systems, software, design, processes, and methods. The healthcare industry has seen a decrease in production and development expenditures as a result of the patents on some 3D printing technology expiring.
Because of its robust research infrastructure, attractive investment climate, and sophisticated healthcare systems, North America is poised to lead the world in 3D printing healthcare applications in the future. More innovation is anticipated as machine learning and artificial intelligence are incorporated into 3D printing procedures. But there are still issues like complicated regulations and a large upfront cost. Nevertheless, the expanding uses in regenerative medicine, prostheses, and surgical treatments point to a bright future for the North American sector.
Key Factors Driving the North America 3D Printing in Healthcare Market Growth
Rising Demand for Personalized Medical Solutions
Personalized medicine has become a core driver of healthcare innovation in North America. 3D printing enables the creation of customized implants, prosthetics, and surgical instruments tailored to individual patient anatomy. This level of personalization improves patient outcomes, reduces the risk of complications, and enhances recovery times. Healthcare providers increasingly prefer patient-specific devices over standardized solutions, particularly in orthopedic and dental procedures. Furthermore, advancements in imaging technologies such as CT and MRI allow accurate modeling, which can be seamlessly integrated into 3D printing systems. As healthcare moves toward more precision-based treatments, the role of 3D printing in providing unique, patient-centered solutions will continue to expand, making it a vital contributor to improved efficiency, cost reduction, and enhanced patient satisfaction across North America’s advanced healthcare systems.Advancements in Biocompatible Materials and Medical Devices
The development of new biocompatible materials is driving the adoption of 3D printing in healthcare. These materials, ranging from polymers to bioresorbable composites, allow the safe creation of implants, surgical guides, and prosthetics that integrate effectively with human tissues. The expanding variety of FDA-approved biocompatible materials has opened new opportunities in areas such as bone regeneration, dental implants, and customized surgical instruments. Additionally, the ability to produce complex geometries and lightweight structures through additive manufacturing has led to enhanced medical device performance. North America’s strong research and development ecosystem ensures continuous innovation, fostering further adoption. Medical professionals increasingly rely on these advancements for both functional and aesthetic purposes, from creating natural-looking dental crowns to durable orthopedic implants, thereby fueling the market’s steady growth.Supportive Healthcare Infrastructure and Research Ecosystem
North America benefits from a strong healthcare infrastructure and extensive research ecosystem, which significantly contribute to the growth of 3D printing in healthcare. Hospitals, universities, and specialized research institutions are investing heavily in advanced printing technologies to enhance surgical planning, medical education, and patient-specific treatment. Funding from government initiatives and private investments accelerates adoption across healthcare facilities. Additionally, the region’s favorable regulatory framework, including FDA approvals for 3D-printed medical devices, has encouraged broader market confidence. The growing number of collaborations between technology providers and medical professionals ensures the continuous integration of innovative solutions into healthcare practices. This environment not only supports innovation but also positions North America as a leader in pioneering applications such as bio-printing tissues and organs, providing a strong platform for market growth in the coming years.Challenges in the North America 3D Printing in Healthcare Market
High Initial Costs and Limited Accessibility
One of the primary challenges in adopting 3D printing in healthcare is the high initial investment required for advanced equipment and materials. Many healthcare institutions, particularly small and mid-sized facilities, find it difficult to allocate budgets for these technologies. Beyond equipment, the costs of training professionals, maintaining machines, and sourcing biocompatible materials add to the financial burden. While larger hospitals and research centers are integrating 3D printing, accessibility remains limited in smaller clinics and rural areas. This cost barrier can slow widespread adoption and create disparities in access to advanced medical care. Addressing this challenge requires more affordable solutions, improved cost-efficiency, and financial support from both public and private sectors to make the benefits of 3D printing in healthcare accessible across all healthcare segments.Regulatory Complexities and Quality Assurance
Regulatory challenges present another barrier to the growth of 3D printing in healthcare. The approval process for 3D-printed medical devices and implants is complex, requiring strict adherence to safety, quality, and biocompatibility standards. This often delays commercialization and adoption across medical facilities. Ensuring consistent quality and reproducibility in customized devices is also a concern, as deviations in printing processes can compromise patient safety. Healthcare providers must comply with stringent guidelines set by regulatory authorities such as the FDA, adding to operational hurdles. Additionally, the rapid pace of innovation in 3D printing often outpaces regulatory frameworks, creating uncertainty for manufacturers and healthcare professionals. Addressing these challenges requires clear guidelines, standardized protocols, and ongoing collaboration between regulators, healthcare providers, and technology developers to ensure safe and efficient integration into the healthcare system.North America 3D Printing in Healthcare Market Overview by Regions
The North America 3D printing in healthcare market is segmented into the United States, Canada, and Mexico, with the U.S. leading adoption due to its advanced healthcare system, supportive regulations, and high research investments. The following provides a market overview by region:United States 3D Printing in Healthcare Market
The United States dominates the North America 3D printing in healthcare market, supported by advanced medical infrastructure, robust research and development, and favorable regulatory frameworks. Hospitals and research institutions are increasingly adopting 3D printing to create customized surgical models, implants, and prosthetics, enhancing precision and reducing costs. The U.S. also benefits from FDA approvals for 3D-printed devices, boosting trust and adoption across healthcare sectors. Rising demand for personalized medicine and technological innovations, including biocompatible materials and bio-printing applications, are fueling market expansion. Furthermore, strong collaborations between medical device manufacturers, technology providers, and academic institutions are driving innovation. However, challenges related to high equipment costs and regulatory complexities persist. Despite this, the U.S. remains at the forefront of global advancements in 3D printing healthcare solutions, providing a foundation for continued growth in applications such as regenerative medicine and surgical planning.Canada 3D Printing in Healthcare Market
Canada’s 3D printing in healthcare market is growing steadily, supported by government initiatives, research collaborations, and adoption in hospitals and clinics. The technology is increasingly being used for surgical planning, dental applications, and the development of patient-specific implants. Universities and research institutions play a pivotal role in advancing innovations, often partnering with technology providers to improve healthcare outcomes. Canadian healthcare facilities are also leveraging 3D printing for medical education, creating anatomical models that enhance training and preoperative simulations. While the country benefits from its robust healthcare system, the market still faces challenges related to high costs and limited accessibility in smaller regions. However, growing awareness of the efficiency, cost reduction, and precision enabled by 3D printing is expanding adoption. With supportive policies and research-driven innovation, Canada is expected to strengthen its role in North America’s healthcare 3D printing landscape.Mexico 3D Printing in Healthcare Market
Mexico is emerging as a promising market for 3D printing in healthcare, driven by increasing investments in medical infrastructure and growing adoption in urban hospitals. The technology is being used in areas such as dentistry, orthopedics, and surgical planning, enabling cost-effective and customized medical solutions. Mexican universities and research institutions are beginning to explore 3D printing applications in medical education and clinical research, fostering innovation. Despite these advancements, the market faces barriers including high equipment costs, limited awareness, and uneven accessibility across rural healthcare facilities. Nevertheless, international collaborations with U.S. and Canadian institutions are helping accelerate knowledge transfer and adoption. As healthcare modernization efforts continue, Mexico is expected to see steady growth in the use of 3D printing for personalized treatments, positioning it as a vital player in the broader North American healthcare 3D printing market.Market Segmentations
Offering
- System
- Materials
- Services
Technology
- Droplet Deposition
- Photopolymerization
- Laser Beam Melting
- Electronic Beam Melting
- Laminated Object Manufacturing
- Others
Application
- Dental
- Wearable Devices
- Prosthetics
- Medical Implants
- Tissue Engineering
- Others
Country
- United States
- Canada
All the Key players have been covered
- Overviews
- Key Persons
- Recent Developments
- SWOT Analysis
- Revenue Analysis
Company Analysis:
- 3D Systems Corporation
- Exone Company
- Formlabs Inc.
- General Electric
- Materialise NV
- Oxferd Performance Materials, Inc.
- Organovo Holdings, Inc.
Table of Contents
Companies Mentioned
- 3D Systems Corporation
- Exone Company
- Formlabs Inc.
- General Electric
- Materialise NV
- Oxferd Performance Materials, Inc.
- Organovo Holdings, Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2025 |
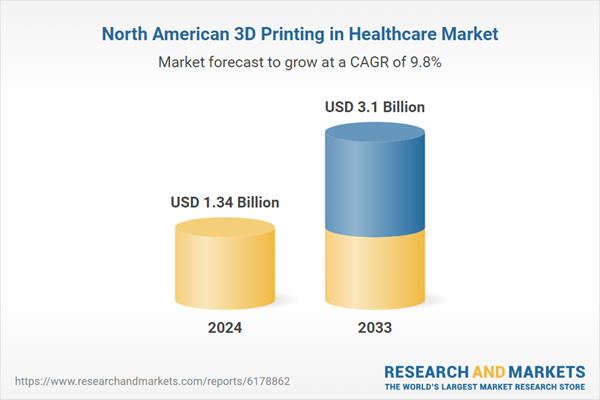
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 1.34 Billion |
| Forecasted Market Value ( USD | $ 3.1 Billion |
| Compound Annual Growth Rate | 9.7% |
| Regions Covered | North America |
| No. of Companies Mentioned | 7 |