Neonatal Intensive Care (NIC) is a specific field of health care that deals with ill or medically needy newborns, particularly premature babies or babies born with congenital conditions. The care is given in a Neonatal Intensive Care Unit (NICU), which is equipped with sophisticated technology and staffed by specially trained specialists, such as neonatologists and nurses. NICUs are important in assisting the health and development of vulnerable newborns through monitoring, ventilatory assistance, medication, and customized nutritional requirements. The aim is to stabilize their condition and foster growth so that they can develop well once discharged.
The need for neonatal intensive care has increased because of advances in medical technology and practices, resulting in increased survival rates for preterm babies. While families can be overwhelmed by the NICU, the high level of specialized care can profoundly enhance health results. Growing public awareness has highlighted the significance of NICUs and the necessity for continued research and funding for this life-saving area.
Top Companies in Global Neonatal Intensive Care
Masimo Corporation
Formation: 1989
Headquarters: Irvine, California
Masimo Corp, headquartered in Irvine, California, deals in medical technology under the specialization of non-invasive patient monitoring sensors and devices to provide better patient care. Its primary technologies are Signal Extraction Technology (SET) and the rainbow SET platform. Masimo deals in a variety of products such as patient monitors, pulse oximeters, specialty sensors, and remote alarms. It sells its products worldwide through multiple channels to consumers, emergency services, home healthcare providers, and hospitals. It is well established in the Americas, Europe, the Middle East, Africa, Asia, and Australia.
3M Company
Foundation: 1902
Headquarters: United States of America
3M Company is an international manufacturer and distributor of a broad portfolio of industrial products and solutions such as advanced materials, home care, office supplies, and personal safety products. 3M also provides medical and dental care, consumer health, food safety, and health information systems solutions to different industries such as automotive, electronics, and healthcare. 3M is headquartered in St. Paul, Minnesota, with manufacturing sites in the Americas, Asia Pacific, Europe, the Middle East, and Africa.
Medtronic PLC
Foundation: 1949
Headquarter: Ireland
Revenue: US$32.4 Billion in 2023
Medtronic plc is a worldwide medical technology firm that manufactures, designs, develops, and markets medical devices and solutions. It produces products for the treatment of heart valve disease, heart failure, coronary artery disease, vascular disease, neurological diseases, and musculoskeletal conditions as well as biological products for use in orthopedic and dental procedures. Medtronic distributes its products to hospitals, healthcare practitioners, clinics, government programs, and distributors in Asia Pacific, Europe, the Americas, the Middle East, and Africa.
Koninklijke Philips N.V
Year of establishment: 1891
Headquartered: Netherlands
Koninklijke Philips NV (Philips) is a technology company that manufactures and designs medical systems and consumer electronics. Its areas of interest are precision diagnosis, image-guided therapy, monitoring, and personal health. Products are X-ray, MRI, and CT systems, hospital monitoring systems, and consumer items such as toothbrushes and hair clippers. Philips provides solutions to healthcare providers, patients, and consumers in Europe, North America, and Asia.
Siemens Healthcare GmbH
Foundation: 2014
Headquarters: Germany
Siemens Healthineers AG is a subsidiary of Siemens AG, a medical technology corporation that develops and produces diagnostic and therapeutic products, such as imaging equipment and in vitro diagnostics. Molecular imaging, X-ray, ultrasound, computed tomography, magnetic resonance imaging systems, and point-of-care testing are its primary products. It has a wide customer base across hospitals, laboratories, and pharmaceuticals, and is globally represented in Europe, the Americas, the Middle East, Africa, and the Asia-Pacific region.
Terumo Corporation Sustainability News
In February 2025, Terumo Corporation announced it has been named an "A-List" company in the "Water Security" sector by the global non-profit CDP for 2024. Further, in April 2023, Terumo Corporation announced that its science-based targets to reduce greenhouse gas (GHG) emissions have been approved by the Science Based Targets initiative (SBTi) as being consistent with the world's goal of capping the temperature rise at 1.5°C above pre-industrial levels.
Drägerwerk AG & Co. KGaA Sustainability News
Drägerwerk AG & Co. KGaA has achieved outstanding progress in its green efforts, managing to reduce its CO2 emissions by a commendable 31 percent from the baseline year of 2015. With a broad and ambitious vision, the company is already on track to meet its major goal of cutting emissions by a full one-third by 2025. Even further ahead, Drägerwerk is determined to cut its carbon footprint in half by 2030, leading the industry in sustainability. The long-term aspiration is reaching climate neutrality in 2045, underscoring the company's commitment to developing a healthier world for future generations.
Cardinal Health Company Recent Development
Aug 2023, Cardinal Health launched its next-generation NTrainer™ System 2.0, a medical device intended to enable premature and newborn infants to acquire the oral coordination skills necessary for earlier transition to independent feeding, thereby supporting a shortened neonatal intensive care unit (NICU) length of stay. By assessing in real-time, the NTrainer™ System offers clinicians the objective data necessary to monitor the progress of an infant in building up pre-feeding skills, with parents having confidence in their infants' progress and potential to succeed upon discharge.
ICU Medical Company Recent Development
Nihon Kohden OrangeMed, Inc. was cleared in June 2023 by the U.S. Food and Drug Administration for its NKV-440 Ventilator System. The NKV-440 is an air turbine-driven ventilator that accommodates a broad patient population from neonates to adults. It is compressed air-independent and achieves high-quality performance that is appropriate for intensive care units in a more compact and mobile configuration. This ventilator also boasts the same app-based, clinician-oriented user interface as the NKV-550 Series Ventilator System.
Inspiration Healthcare Group plc Recent Development
November 2023, Inspiration Healthcare Group plc (AIM: IHC), a pioneer in medical technology with expertise in neonatal intensive care, has released the SLE1500, the latest addition to its family of dedicated neonatal ventilators. The SLE1500 is presented as a portable respiratory support system with non-invasive modes of ventilation, especially created for the tiniest and most fragile neonatal patients who need respiratory support. This new device seeks to offer vital support while protecting the fragile lung health of these vulnerable infants.
Phoenix Medical Systems Company SWOT Analysis
Strengths: Strong Product Portfolio and Market Expertise in Neonatal Care - Phoenix Medical Systems is a well-known manufacturer specializing in equipment for neonatal and maternal care. The company has a broad range of products under its belt, comprising infant warmers, phototherapy equipment, incubators, and resuscitators. Focusing on quality, safety, and affordability, Phoenix has become a reputable brand with hospitals, maternity clinics, and healthcare centers. Among the strengths of the company is its thorough familiarity with neonatal care requirements, especially in developing economies where access to cost-effective, reliable, and accessible healthcare solutions is critical. Through the incorporation of easy-to-use technology and international quality standards, Phoenix has improved its standing in the market. Moreover, Phoenix Medical Systems has a robust research and development (R&D) culture that enables it to design cutting-edge solutions for different types of healthcare environments. It also has a wide service network in India and internationally, which promotes continuous support for the customers and boosts the reliability of the product and customer satisfaction. This combination of technical capabilities, cost-effectiveness, and reliability gives Phoenix Medical Systems an edge in the neonatal intensive care market.
Utah Medical Products Company SWOT Analysis
Strengths: Strong Reputation and High-Quality Product Line in Neonatal Care - Utah Medical Products has built a robust image of offering accurate, durable, and clinically efficient medical devices, especially in obstetrics, gynecology, neonatal intensive care, and other special healthcare. The company's product line for neonatal features products like umbilical catheterization equipment, disposable pressure transducers, and neonatal suction control systems, all showing high clinical safety and patient outcome focus. One of the strengths of Utah Medical Products is its unrelenting commitment to quality and innovation. Every product is designed and produced according to stringent U.S. These adhere to FDA and ISO standards, providing high reliability and predictable performance. The firm uses lean manufacturing techniques and keeps production capacity in-house, permitting close control over quality and supply chains. Utah Medical Products has developed long-term relationships with healthcare professionals and hospitals, which increases brand loyalty and trust. Its capability to provide clinically tested, long-lasting, and user-friendly neonatal devices makes it a go-to partner for neonatal care units everywhere in the world. The right balance of meticulous product development, rigorous quality checks, and ongoing innovation gives Utah Medical Products an edge in the neonatal intensive care space.
Vyaire Medical Company SWOT Analysis
Strengths: Excellent Expertise in Respiratory and Ventilation Solutions for Neonates - Vyaire Medical's strongest asset in the neonatal intensive care market is its deep focus on respiratory therapy and ventilation systems. The firm's product line features sophisticated neonatal ventilators, oxygen therapy devices, and monitoring solutions specifically for critical infant patients. These products are recognized for precision, safety, and clinical flexibility, which are critical in the case of preterm and critically ill infants. Vyaire's extensive history of respiratory innovation provides it with a unique competitive edge since neonatal ventilation necessitates advanced pressure control and real-time monitoring functions. Its adherence to evidence-based design and partnership with neonatologists assures that its products are of high clinical quality. The company's international footprint and established hospital and NICU specialist relationships enhance its market penetration and credibility. In addition, Vyaire's emphasis on digital integration - e.g., connectivity between ventilators and patient monitoring platforms - improves workflow efficiency and patient safety. This clinical and technology alignment makes Vyaire a reliable and innovative partner for the neonatal care segment.
Global Virtual Reality Market
- Historical Trends
- Forecast Analysis
Company Analysis
Overview
- Company History and Mission
- Business Model and Operations
- Workforce
- Executive Leadership
- Operational Management
- Division Leaders
- Board Composition
- Mergers & Acquisitions
- Partnerships
- Investments
- Renewable Energy Adoption
- Energy-Efficient Infrastructure
- Use of Sustainable Packaging Materials
- Water Usage and Conservation Strategies
- Waste Management and Circular Economy Initiatives
- Product Profile
- Quality Standards
- Product Pipeline
- Product Benchmarking
- Strengths
- Weaknesses
- Opportunities
- Threats
The above information will be available for all the following companies:
- Masimo Corporation
- 3M Company
- Medtronic PLC
- Koninklijke Philips N.V
- Siemens Healthcare Gmbh
- Terumo Corporation
- Angio Dynamics
- Drägerwerk AG & Co. KGaA
- GE Healthcare
- Fisher & Paykel Healthcare
- BD (Becton, Dickinson and Company)
- Natus Medical Incorporated
- Vyaire Medical
- Atom Medical Corporation
- Utah Medical Products
- Cardinal Health
- ICU Medical
- Nihon Kohden Corporation
- Phoenix Medical Systems
- Inspiration Healthcare Group Plc
Table of Contents
1. Neonatal Intensive Care Market1.1 Historical Trends
1.2 Forecast Analysis
2. Market Share Analysis
3. Masimo Corporation
3.1 Overview
3.1.1 Company History and Mission
3.1.2 Business Model and Operations
3.1.3 Workforce
3.2 Key Persons
3.2.1 Executive Leadership
3.2.2 Operational Management
3.2.3 Division Leaders
3.2.4 Board Composition
3.3 Recent Development & Strategies
3.3.1 Mergers & Acquisitions
3.3.2 Partnerships
3.3.3 Investments
3.4 Sustainability Analysis
3.4.1 Renewable Energy Adoption
3.4.2 Energy-Efficient Infrastructure
3.4.3 Use of Sustainable Packaging Materials
3.4.4 Water Usage and Conservation Strategies
3.4.5 Waste Management and Circular Economy Initiatives
3.5 Product Analysis
3.5.1 Product Profile
3.5.2 Quality Standards
3.5.3 Product Pipeline
3.5.4 Product Benchmarking
3.6 Strategic Assessment: SWOT Analysis
3.6.1 Strengths
3.6.2 Weaknesses
3.6.3 Opportunities
3.6.4 Threats
3.7 Revenue Analysis
Companies Mentioned
- Masimo Corporation
- 3M Company
- Medtronic PLC
- Koninklijke Philips N.V
- Siemens Healthcare Gmbh
- Terumo Corporation
- Angio Dynamics
- Drägerwerk AG & Co. KGaA
- GE Healthcare
- Fisher & Paykel Healthcare
- BD (Becton, Dickinson and Company)
- Natus Medical Incorporated
- Vyaire Medical
- Atom Medical Corporation
- Utah Medical Products
- Cardinal Health
- ICU Medical
- Nihon Kohden Corporation
- Phoenix Medical Systems
- Inspiration Healthcare Group Plc
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2025 |
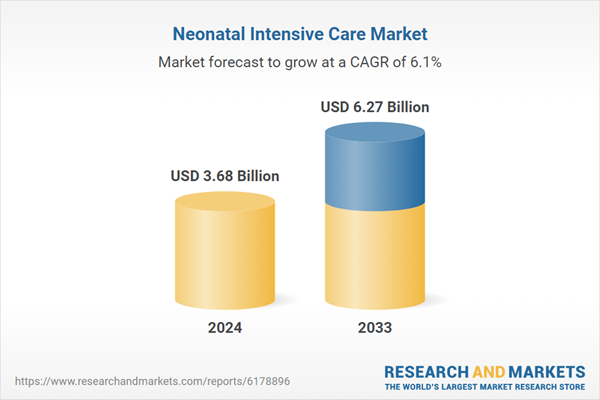
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 3.68 Billion |
| Forecasted Market Value ( USD | $ 6.27 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |