A catheter is a thin, flexible medical tube that is placed inside the body to access internal organs and blood arteries, or to deliver or remove gasses, fluids, or drugs. Numerous medical procedures, such as neurological, gastrointestinal, urinary, and cardiovascular treatments, employ catheters. Heart catheters, intravenous (IV) catheters, and urine catheters are common varieties. Depending on the use, they are made of materials like silicone, latex, or polyurethane and can be used for a short or lengthy period of time. Because they provide minimally invasive operations, effective fluid management, and ongoing physiological function monitoring, catheters are essential to patient care.
The rising incidence of chronic illnesses such kidney disease, urinary tract infections, and cardiovascular ailments is driving the catheter market's expansion. The market is expanding due to factors such growing geriatric populations, technological breakthroughs in catheter design, and rising desire for less invasive procedures. Material advancements like biocompatible and antibacterial coatings improve patient safety and lower the risk of infection. Demand is further increased by growing surgery volumes, expanding healthcare infrastructure, and more knowledge of cutting-edge treatment choices. Additionally, the use of new, single-use, and specialty catheters is being encouraged globally by the rising rate of hospital-acquired infections and the acceptance of home healthcare.
Top Companies in Catheter Industry
Boston Scientific Corporation
Establishment: 1979Headquarters: United States of America
Devices for a range of interventional medical specialties are developed, manufactured, and marketed by Boston Scientific Corp. (Boston Scientific), a medical technology company. Among the industries in which the company sells its products are electrophysiology, gastroenterology, gastrointestinal surgery, female pelvic medicine, gynecology, interventional cardiology, interventional radiology, neurological surgery, orthopedic surgery, pain management, pulmonology, urology, and vascular surgery. Boston Scientific serves hospitals, clinics, outpatient facilities, and doctor's offices all around the world. The company has production facilities in the United States, Ireland, Costa Rica, Brazil, Malaysia, and Puerto Rico. It offers its products directly as well as through a network of distributors and dealers across Europe, the Middle East, Africa, Asia Pacific, and the Americas. The headquarters of Boston Scientific are located in the US city of Marlborough, Massachusetts.
Teleflex Incorporated
Establishment: 1943Headquarters: United States of America
Medical equipment are produced by Teleflex Inc. (Teleflex). It primarily designs, develops, manufactures, and distributes single-use medical devices for use in critical care and surgery. Its primary products are respiratory care items, anesthetic supplies, interventional catheters, and vascular access devices. The company also offers custom orders, partial shipments, clinical support services, technical support, and product maintenance. Teleflex sells its products to hospitals, healthcare providers, distributors, and original equipment manufacturers under a variety of names, such as Arrow, Deknatel, LMA, Pilling, QuikClot, Rusch, UroLift, and Weck. The company markets and sells its products through direct sales and distributors. It operates manufacturing facilities in the Czech Republic, Malaysia, Mexico, and the United States. Teleflex's headquarters are located in Wayne, Pennsylvania, in the United States.
Medtronic Plc.
Establishment: 1949
Headquarters: Ireland
Medtronic plc (Medtronic) is a medical technology company that designs, develops, manufactures, and markets medical devices and solutions. The company's main activities include the design, development, manufacturing, and marketing of biomedical engineering products. It offers products to treat conditions of the spine, musculoskeletal system, ears, nose, and throat, as well as heart valve issues, heart failure, aortic, peripheral vascular, venous, renal, and neurological diseases. Furthermore, Medtronic provides biologic solutions for the orthopedic and dental sectors. The company offers its products to hospitals, clinics, government healthcare programs, third-party healthcare providers, distributors, and group purchasing groups across Asia Pacific, Europe, the Americas, the Middle East, and Africa. Dublin, Ireland is home to Medtronic's headquarters.
Johnson & Johnson
Establishment: 1886Headquarters: United States of America
Johnson & Johnson (J&J) is a healthcare company that conducts research, develops, produces, and distributes innovative drugs and medical technologies. The corporation carries out its operations through its operating companies. It provides pharmaceutical products for treatment areas related to immunological disorders, cancer, neurological disorders, infectious, cardiovascular, and metabolic diseases, as well as medical equipment for use in the fields of cardiovascular, orthopedic, neurovascular, general surgery, and vision care. The company sells its products to merchants, medical professionals, distributors, and hospitals. It runs production facilities across the Western Hemisphere (apart from the US), Africa, Asia-Pacific, Europe, Latin America, and the United States. The headquarters of J&J are located in the US city of New Brunswick, New Jersey.
Edwards Lifesciences Corporation
Establishment: 1958Headquarters: United States of America
Medical technology company Edwards Lifesciences Corp. (Edwards Lifesciences) develops, manufactures, and markets devices for postoperative monitoring, critical care, and structural heart disease. The products offered by the company include transcatheter cardiac valves, hemodynamic monitoring devices, surgical valve replacement and repair devices, pressure monitoring systems, and related instruments and accessories. Among the treatments that make use of its products are transcatheter aortic valve replacement (TAVR), blood conservation, infection control, enhanced postoperative recovery, and the management of sepsis, hypotension, and clotting. The company markets its products directly to customers in addition to having a network of independent distributors in North America, Europe, and Asia-Pacific. Irvine, California is home to Edwards Lifesciences' US headquarters.
SWOT Analysis of Catheter Market
Hollister Incorporated
Strength: Established Reputation in Urological Care - Hollister Incorporated has established a solid reputation as a reliable supplier of urological care products. Strong patient and healthcare professional loyalty has been cultivated by the company's emphasis on patient-centered design, safety, and comfort in its catheter devices. Its commitment to high-quality production, exacting testing, and ongoing development strengthens consumer trust in its goods. Hollister is able to achieve a premium position in the market thanks to its excellent brand recognition, which also helps them maintain a consistent consumer base. Hollister can stand out in a crowded market and guarantee steady demand in both hospital and homecare settings by capitalizing on its reputation.Opportunity: Expansion in Emerging Markets - Hollister has a significant chance to grow by increasing its footprint in developing nations where modern urology care is becoming more widely known and healthcare infrastructure is growing. There is frequently an unmet need for dependable, high-quality catheter options in these areas. Hollister can become a reputable brand by launching its products through collaborations with regional distributors, training programs for medical professionals, and patient awareness campaigns. In addition to satisfying these markets' expanding healthcare demands, this expansion can boost the company's global presence, diversify revenue sources, and raise brand awareness.
Cook Medical Inc.
Strength: Innovative Product Portfolio - A well-known company for its creative approach to creating catheter solutions and other medical devices is Cook Medical Inc. The business continuously makes investments in R&D to produce cutting-edge solutions that increase patient safety, improve procedural accuracy, and expedite clinical workflows. Cook Medical exhibits leadership in medical innovation by incorporating cutting-edge features and materials into their products. By placing a significant emphasis on technological progress, the company not only improves its competitive position but also gains the respect of medical professionals and organizations that place a high value on accuracy, dependability, and better results from urological procedures.Opportunity: Strategic Partnerships for Market Expansion - Cook Medical has a significant opportunity to expand its market reach through strategic partnerships and collaborations. By collaborating with other medical technology companies, healthcare providers, or regional distributors, Cook Medical can broaden its product availability, introduce complementary innovations, and penetrate new markets more effectively. These partnerships also allow for co-development of new solutions and faster adoption of cutting-edge technologies, enhancing the company’s product portfolio and brand presence. Such strategic alliances can accelerate growth, increase market share, and position Cook Medical as a leading and collaborative force in the global catheter market.
Sustainability Analysis of Catheter Market
Abott Laboratories
Through its 2030 Sustainability Plan, Abbott Laboratories incorporates sustainability into its worldwide strategy, placing a strong emphasis on resource efficiency, climate action, ethical sourcing, and fair access to healthcare. By 2030, the corporation wants to cut its absolute Scope 1 and 2 greenhouse gas emissions by 30% from 2018 levels; as of 2023, it has already reduced them by 7%. Abbott also places a great priority on water stewardship, especially at its operations in high-risk areas, where it has many Alliance for Water Stewardship-certified locations. 53 sites have attained zero-waste-to-landfill status, and 91% of operating waste is diverted from landfills in waste management. The company has worked closely with suppliers to ensure ethical and ecologically responsible procurement, and has introduced more than 40 sustainable packaging projects to improve recyclability and reduce material use. Beyond environmental goals, Abbott’s sustainability strategy includes social impact, aiming to improve global health by reaching 3 billion people annually with affordable medical technologies and nutrition solutions by 2030, reflecting its holistic ESG commitment.Recent Development in Catheter Industry
- Stryker Corporation paid USD 4.9 billion to acquire Inari Medical, Inc. in February 2025. This move strengthened Stryker's peripheral vascular position in the face of significant segment growth and enabled the company to offer cutting-edge treatments for eliminating venous thromboembolism (VTE) clots.
- The newly developed Clik-FIX Epidural/Peripheral Nerve Block (PNB) Catheter Securement Device was introduced by B. Braun Medical Inc. in January 2025. This new member of the Clik-FIX family is intended to be safe, discrete, and soft. During regional anesthetic operations, it seeks to lower the risk of catheter displacement and dislodgement. The gadget is designed for easy use and dependable security, tackling issues that patients and medical professionals encounter.
- Terumo Corporation subsidiary Terumo Interventional Systems (TIS) further expanded its radial-to-peripheral (R2P) range in December 2024 with the announcement of the R2P NaviCross Peripheral Support Catheter's U.S. launch. The catheter, which comes in a 200-cm length, is made of stainless steel and double braided to provide it excellent trackability and torque control, making it easier to traverse lesions during intricate procedures.
- For USD 4.2 billion, Becton, Dickinson and Company (BD) purchased the Critical Care product from Edwards Lifesciences in September 2024. With the addition of the well-known Swan-Ganz pulmonary artery catheters, this acquisition enhanced BD's portfolio of smart linked care solutions.
- Stryker Corporation introduced the AXS Vecta 46 Intermediate Catheter in Korea, Japan, and the United States in July 2023. Stryker's dedication to growing its product line and footprint in important global neurovascular markets is demonstrated by its Neurovascular division launch.
Company Analysis Format
Catheter Market & Forecast
- Historical Trends
- Forecast Analysis
Market Share Analysis - Catheter Market
Company Analysis-Abbott Laboratories
Overview
- Company History and Mission
- Business Model and Operations
- Workforce
Key Persons
- Executive Leadership
- Operational Management
- Division Leaders
- Board Composition
Recent Development & Strategies
- Mergers & Acquisitions
- Partnerships
- Investments
Sustainability Analysis
- Renewable Energy Adoption
- Energy-Efficient Infrastructure
- Use of Sustainable Packaging Materials
- Water Usage and Conservation Strategies
- Waste Management and Circular Economy Initiatives
Product Analysis
- Product Profile
- Quality Standards
- Product Pipeline
- Product Benchmarking
Strategic Assessment: SWOT Analysis
- Strengths
- Weaknesses
- Opportunities
- Threats
Revenue Analysis
The above information will be provided for all the following companies:
- Becton Dickinson and Company
- Boston Scientific Corporation
- Teleflex Incorporated
- Medtronic Plc.
- Johnson & Johnson
- Edwards Lifesciences Corporation
- Stryker Corporation
- Hollister Incorporated
- B. Braun Melsungen AG
- Cook Medical Inc.
- Terumo Corporation
- ConvaTec Group PLC
- AngioDynamics Inc.
- Smiths Medical
Table of Contents
1. Global Catheter Market1.1 Historical Trends
1.2 Forecast Analysis
2. Market Share Analysis
3. Becton Dickinson and Company
3.1 Overview
3.1.1 Company History and Mission
3.1.2 Business Model and Operations
3.1.3 Workforce
3.2 Key Persons
3.2.1 Executive Leadership
3.2.2 Operational Management
3.2.3 Division Leaders
3.2.4 Board Composition
3.3 Recent Development & Strategies
3.3.1 Mergers & Acquisitions
3.3.2 Partnerships
3.3.3 Investments
3.4 Sustainability Analysis
3.4.1 Renewable Energy Adoption
3.4.2 Energy-Efficient Infrastructure
3.4.3 Use of Sustainable Packaging Materials
3.4.4 Water Usage and Conservation Strategies
3.4.5 Waste Management and Circular Economy Initiatives
3.5 Product Analysis
3.5.1 Product Profile
3.5.2 Quality Standards
3.5.3 Product Pipeline
3.5.4 Product Benchmarking
3.6 Strategic Assessment: SWOT Analysis
3.6.1 Strengths
3.6.2 Weaknesses
3.6.3 Opportunities
3.6.4 Threats
3.7 Revenue Analysis
Companies Mentioned
- Becton Dickinson and Company
- Boston Scientific Corporation
- Teleflex Incorporated
- Medtronic Plc.
- Johnson & Johnson
- Edwards Lifesciences Corporation
- Stryker Corporation
- Hollister Incorporated
- B. Braun Melsungen AG
- Cook Medical Inc.
- Terumo Corporation
- ConvaTec Group PLC
- AngioDynamics Inc.
- Smiths Medical
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2025 |
| Forecast Period | 2024 - 2033 |
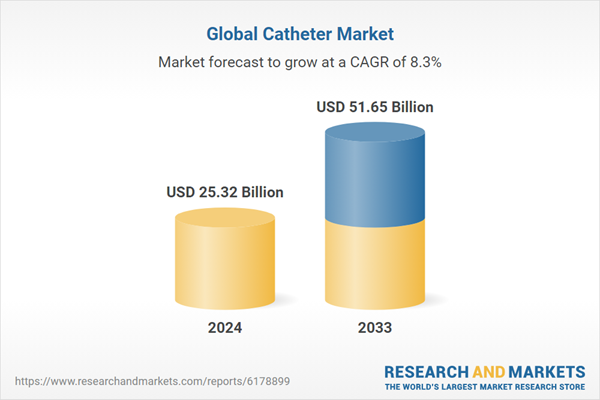
| Estimated Market Value ( USD | $ 25.32 Billion |
| Forecasted Market Value ( USD | $ 51.65 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |