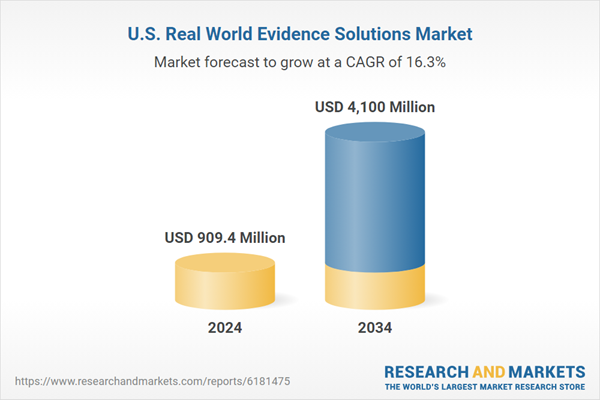
The robust growth is fueled by increased emphasis on expediting drug development, lowering costs, and enhancing post-market safety and efficacy evaluation. Stakeholders are placing greater reliance on real-world evidence to guide reimbursement strategies and clinical decision-making. RWE solutions gather, analyze, and interpret data from everyday healthcare environments such as electronic health records, claims databases, registries, and wearable devices to derive clinical insights beyond conventional trial settings. As payers, regulators, and clinicians demand more real-time evidence, the role of RWE is becoming critical throughout the lifecycle of medical products. This dynamic shift toward evidence-driven strategies, plus growing investments in analytics, is reshaping how drugs and devices are developed, approved, and monitored in the U.S. market.
In 2024, the services segment held a 58.4% share. Its leading position is supported by the widespread offering of subscription models and advanced analytics platforms, and by growing uptake of RWE services among life sciences firms. These services encompass study planning, data integration, quality control, and regulatory support. They harmonize diverse data streams from health records to wearable outputs to enable robust real-world studies that meet compliance standards and decision-making needs.
The drug development and approvals segment is expected to reach USD 1.6 billion by 2034. This growth is driven by increased clinical trial activity, the need to optimize trial designs, and the use of RWE to support regulatory submissions and accelerate approvals. The segment includes protocol formulation, patient recruitment, trial optimization, safety and efficacy monitoring, and evidence generation for regulatory bodies.
The pharmaceutical and medical device companies segment held a 60.4% share in 2024. These organizations are the primary adopters of RWE tools and platforms, deploying them across product lifecycle stages. RWE helps them pinpoint patient cohorts, refine trial protocols, and produce real-world data aligned with regulatory and market access demands, either supplementing or, in some cases, substituting traditional randomized controlled trials.
Prominent participants in the U.S. Real World Evidence Solutions Market include ICON plc, Oracle Corporation, Aetion, Inc., TriNetX, Cytel Inc., Merative, Flatiron Health Inc., Tempus, Syneos Health Inc., Medidata Solutions, Inc., Thermo Fisher Scientific, Inc., UnitedHealth Group Incorporated, IQVIA Holdings Inc., Parexel International Corporation, and Fortrea Holdings Inc. Firms in the U.S. real world evidence solutions market are pursuing several strategic initiatives to solidify their market position. Many are investing heavily in AI and machine learning to improve predictive modeling, causal inference, and data analytics capabilities. Partnerships and alliances with healthcare systems, payers, and research institutions are helping to access richer, more diverse real-world datasets. Acquisitions and mergers are being used to broaden service offerings, add novel technology, or expand geographic reach.
Comprehensive Market Analysis and Forecast
- Industry trends, key growth drivers, challenges, future opportunities, and regulatory landscape
- Competitive landscape with Porter’s Five Forces and PESTEL analysis
- Market size, segmentation, and regional forecasts
- In-depth company profiles, business strategies, financial insights, and SWOT analysis
This product will be delivered within 2-4 business days.
Table of Contents
Companies Mentioned
The companies profiled in this U.S. Real World Evidence Solutions market report include:- Aetion, Inc.
- Cytel Inc
- Flatiron Health Inc
- Fortrea Holdings Inc
- IBM Corporation
- ICON plc
- IQVIA Holdings Inc
- Medidata Solutions, Inc.
- Merative
- Oracle Corporation
- Parexel International Corporation
- Syneos Health Inc
- Tempus
- TriNetX
- Thermo Fisher Scientific, Inc.
- UnitedHealth Group Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 90 |
| Published | October 2025 |
| Forecast Period | 2024 - 2034 |
| Estimated Market Value ( USD | $ 909.4 Million |
| Forecasted Market Value ( USD | $ 4100 Million |
| Compound Annual Growth Rate | 16.3% |
| Regions Covered | Global, United States |
| No. of Companies Mentioned | 17 |