Semaglutide Market
Semaglutide - a long-acting GLP-1 analog - has become the reference therapy across type-2 diabetes and prescription anti-obesity categories, expanding from weekly injections to higher-dose obesity indications and oral formulations. Its market power stems from clinically validated outcomes (glycemic control, weight loss, risk reduction surrogates), broad prescriber familiarity, and lifecycle management spanning pens, autoinjectors, and tablets. Demand consistently outpaces supply, pushing upstream investments in peptide synthesis (resin, solvents), sterile fill-finish, device components, and cold-chain logistics; brand owners increasingly ration by indication and geography while scaling capacity. Payer behavior is the key commercial swing factor: diabetes coverage is entrenched, while obesity coverage varies widely by employer, plan design, and HTA rulings, shaping out-of-pocket exposure and adherence. Real-world data and label expansions continue to enlarge the addressable base beyond early adopters, supporting longer treatment duration and higher persistence with structured dose-escalation and side-effect management. Competitive intensity rises from next-gen incretin agents (dual/triple agonists) and alternative delivery formats, yet semaglutide retains durable share where continuity of care, supply reliability, and established dosing algorithms matter. Downstream, telehealth, specialty pharmacy, and hub services orchestrate prior authorization, patient onboarding, and injection training; employer programs and cardiometabolic bundles become influential channels. Over the medium term, manufacturing de-bottlenecking, oral dose innovation, and outcomes-based contracts will determine volume elasticity, while policy scrutiny (advertising, compounding, waste) and safety/contraindication management shape reputation and payer posture. Net-net, semaglutide has transitioned from a high-impact diabetes drug to a multi-indication franchise with ecosystem-level influence on primary care, obesity medicine, and cardiometabolic prevention.Semaglutide Market Key Insights
- Dual-franchise engine. Diabetes scripts provide durable baseline volume; obesity indications add a faster-growing, high-visibility second engine, with distinct payer rules, dose strengths, and service models.
- Supply is strategy. Peptide capacity, lyophilization, and pen component availability remain gating; winners prove reliability via dual sourcing, regional fill-finish, safety stocks, and transparent allocation policies.
- Access is the swing factor. Diabetes coverage is standardized; obesity access depends on employer benefits, step edits, and country HTA outcomes. Copay structures and prior authorization workflows drive initiation and persistence.
- Oral lifecycle matters. Oral semaglutide broadens prescriber reach and patient preference, especially in primary care and needle-averse segments, while higher doses target obesity-adjacent needs.
- Care model redesign. Telehealth plus specialty pharmacy hubs compress time-to-therapy, manage titration and side effects, and improve refill cadence; integrated cardiometabolic clinics emerge as anchor channels.
- Competitive pressure from next-gen incretins. Dual/triple agonists reset efficacy expectations; semaglutide defends share via outcomes evidence, tolerability familiarity, and device/route choice.
- Persistence over initiation. GI tolerability, dose-escalation coaching, nutrition/behavioral support, and clear expectations around plateaus are critical to maintaining therapy beyond early cycles.
- Economics beyond drug price. Employers assess absenteeism, cardiometabolic risk costs, and bariatric deferral; payers trial outcomes-based agreements and utilization caps to balance budgets.
- Policy and reputation management. Advertising scrutiny, compounding controversies, and environmental waste from pens drive stewardship programs (safe disposal, training, controlled messaging).
- Globalization with local nuance. Label scope, obesity recognition in guidelines, and pharmacy channel structure vary widely; success requires country-specific access playbooks and clinician education.
Semaglutide Market Reginal Analysis
North America
Demand is propelled by high diagnosis rates, primary-care adoption, and strong specialist advocacy. Diabetes coverage is broad; obesity coverage remains heterogeneous across commercial plans and public programs, creating a mix of reimbursed and cash-pay cohorts. Telehealth platforms, employer benefits, and specialty pharmacy hubs shape initiation speed and refill reliability. Supply prioritization, copay dynamics, and adherence support (titration, side-effect management) drive real-world persistence.Europe
HTA decisions and country-level obesity strategies determine pace of uptake. Diabetes indications are embedded in formularies; obesity access depends on BMI/complication criteria and budget impact analyses. Prescribing often sits in specialist clinics with structured follow-up. Pharmacy channels favor audited dispensing and pen return programs; outcomes registries and stewardship on safe use underpin reputation and renewal.Asia-Pacific
A diverse landscape: advanced markets show strong guideline alignment and rapid specialist adoption; emerging markets balance growing private-pay demand with constrained public budgets. Oral semaglutide supports primary-care reach, while obesity treatment remains concentrated in urban centers. Local device and cold-chain partners are critical to availability; education on dose-escalation and GI management improves continuity.Middle East & Africa
Rising metabolic disease burden and private insurance penetration support uptake, initially in premium private settings and government employee schemes. Access hinges on negotiated tenders, center-of-excellence models, and reliable cold-chain. Clinician education and patient support programs mitigate discontinuation due to tolerability or stock variability.South & Central America
Urban private markets lead adoption, with public-sector access evolving via targeted programs. Currency volatility and import logistics influence continuity; distributors with robust inventory and patient hubs gain share. Diabetes indications anchor volume; obesity uptake grows where employer or supplemental plans reimburse and where prescriber training on titration and monitoring is widespread.Semaglutide Market Segmentation
By Band
- Wegovy
- Rybelsus
- Ozempic
By Route of Administration
- Oral
- Injection
By End-User
- Hospitals Pharmacies
- Retail Pharmacies
- Online Pharmacies
Key Market players
Novo Nordisk, Catalent, Thermo Fisher Scientific, WuXi AppTec, Samsung Biologics, Bachem, CordenPharma, PolyPeptide Group, AmbioPharm, Lonza, Piramal Pharma Solutions, Siegfried, Recipharm, Baxter (fill-finish), Ajinomoto Bio-Pharma ServicesSemaglutide Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Semaglutide Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Semaglutide market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Semaglutide market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Semaglutide market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Semaglutide market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Semaglutide market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Semaglutide value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Semaglutide industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Semaglutide Market Report
- Global Semaglutide market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Semaglutide trade, costs, and supply chains
- Semaglutide market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Semaglutide market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Semaglutide market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Semaglutide supply chain analysis
- Semaglutide trade analysis, Semaglutide market price analysis, and Semaglutide supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Semaglutide market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Novo Nordisk
- Catalent
- Thermo Fisher Scientific
- WuXi AppTec
- Samsung Biologics
- Bachem
- CordenPharma
- PolyPeptide Group
- AmbioPharm
- Lonza
- Piramal Pharma Solutions
- Siegfried
- Recipharm
- Baxter (fill-finish)
- Ajinomoto Bio-Pharma Services
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
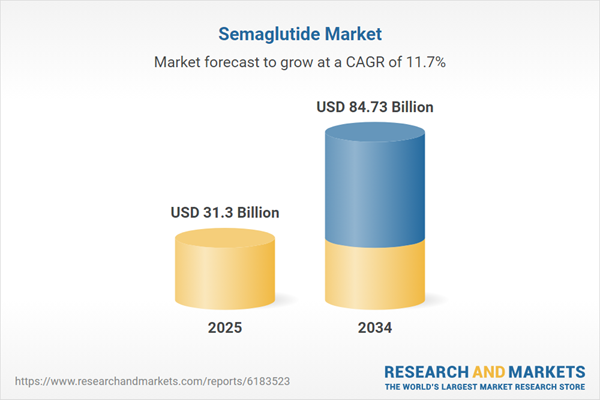
| Estimated Market Value ( USD | $ 31.3 Billion |
| Forecasted Market Value ( USD | $ 84.73 Billion |
| Compound Annual Growth Rate | 11.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |