Duodenoscopes Market
The duodenoscopes market centers on side-viewing endoscopes used primarily for ERCP procedures to diagnose and treat biliary and pancreatic disorders. Core end-uses include stone extraction, stricture dilation, stent placement, sphincterotomy, and tissue sampling across hospitals, tertiary referral centers, and specialized GI clinics. The category is undergoing structural change as infection-control expectations rise: vendors are transitioning from fully reusable designs to platforms with disposable distal caps, semi-disposable components, and fully single-use duodenoscopes. Parallel investment is flowing into reprocessing - automated endoscope reprocessors, sterilants, leak testing, drying cabinets, and digital traceability - to mitigate cross-contamination risk and standardize compliance. On the technology front, high-definition and 4K processors, enhanced imaging (e.g., optical enhancement/virtual chromoendoscopy), improved torque and elevator mechanics, slimmer insertion tubes, and accessory channel durability are differentiators that influence cannulation success and procedure time. Commercial dynamics reflect a mix of capital equipment placements, per-procedure disposables, and service contracts; procurement increasingly weighs lifetime cost of ownership against infection-prevention assurances, uptime, and training support. Competitive intensity remains high with incumbent Japanese and European OEMs, emerging single-use specialists, and ecosystem partners in reprocessing and sterilization. Across regions, procedure volumes track aging demographics, gallstone prevalence, pancreatobiliary oncology, and access to advanced endoscopy. Key headwinds include staffing shortages, credentialing requirements, and sustainability debates around single-use waste; key tailwinds include guideline endorsements for disposable interfaces, growing availability of single-use devices for difficult access or outbreak scenarios, and broader adoption of digital scope tracking. Over the forecast horizon, expect a dual-track market: premium reusable platforms with disposable infection-critical parts, and single-use offerings expanding in high-risk or capacity-constrained settings.Duodenoscopes Market Key Insights
- Infection-control reshapes portfolios. Clinical guidance favoring disposable distal ends and improved reprocessing documentation accelerates product refreshes. Hospitals increasingly adopt scopes with sealed elevators or single-use alternatives to reduce contamination risk during ERCP, especially in immunocompromised cohorts and outbreak responses.
- Single-use gains defined roles. Fully disposable duodenoscopes are gaining traction in select use cases - MDRO exposure, complex isolation protocols, off-hours cases, or centers without robust reprocessing. Cost-benefit narratives hinge on avoided remediation, reduced downtime, and predictable per-case pricing versus reusable capital amortization.
- Reusable platforms remain core. High-volume centers continue to prefer reusable systems for throughput and image quality, but now specify disposable caps, elevator redesigns, and validated cleaning steps. Serviceability, elevator cable robustness, and channel longevity are critical to lifetime ownership costs.
- Imaging and visualization advance. HD/4K processors, enhanced imaging modes, and brighter light sources improve duct visualization, papilla identification, and stone/stent assessment. Ergonomic handle refinements, finer elevator control, and stiffer yet steerable shafts improve cannulation efficiency and reduce procedure time variability.
- Reprocessing workflows professionalize. Investment shifts from manual steps to automated washers, drying cabinets, and digital chain-of-custody platforms. Barcoding/RFID tracking with audit trails supports quality metrics, while competency-based staff training and periodic verification tests limit variability.
- Economics and reimbursement drive adoption. Purchasing committees balance infection risk mitigation with capital budgets. Bundled offerings - scope, processor, accessories, and service - simplify approval; single-use device economics compete where case volumes are modest or remediation costs are high.
- Accessory ecosystem matters. Compatible guidewires, retrieval baskets, sphincterotomes, dilation balloons, and stents impact procedural success. OEMs increasingly curate “optimized” accessory sets and credentialed training pathways to improve first-pass success and reduce fluoroscopy time.
- Data, connectivity, and QA. Integration with hospital IT enables scope history, utilization analytics, and maintenance scheduling. Video capture and structured reporting support peer review and training, while predictive service models reduce unexpected downtime during peak lists.
- Workforce and training constraints. Advanced ERCP skills are concentrated in tertiary centers; vendors differentiate with simulation tools, proctorship programs, and standardized ergonomics to shorten learning curves. Clear IFUs and visual reprocessing aids reduce human-factor error.
- Sustainability vs. safety debate. Environmental impact of single-use devices is weighed against infection prevention. Emerging take-back and recycling pilots, thinner materials, and optimized packaging aim to reduce waste, while reusable platforms tout extended life and repairability alongside disposable critical interfaces.
Duodenoscopes Market Reginal Analysis
North America
Adoption emphasizes infection-prevention assurances and documented reprocessing compliance, with increasing use of disposable distal caps and targeted deployment of single-use scopes in high-risk scenarios. Academic and tertiary centers drive technology upgrades - 4K processors, imaging enhancement, and integrated data capture - supported by vendor training and simulation. Purchasing decisions scrutinize lifetime costs, warranty coverage, and loaner availability to minimize case cancellations. Staffing and credentialing pressures encourage standardized IFUs, automated drying, and digital traceability to pass audits and support quality reporting.Europe
Stringent device and reprocessing standards push hospitals toward scopes with sealed or disposable interfaces and robust post-cleaning drying protocols. Procurement favors validated cleaning workflows, scope tracking, and service programs with rapid turnaround. University hospitals and regional reference centers lead upgrades to high-definition processors and elevator redesigns, while budget-constrained sites pursue retrofit options and extended service plans. Environmental considerations shape debate on single-use adoption, with pilot projects balancing safety and sustainability.Asia-Pacific
Rising ERCP capacity in China, India, Southeast Asia, and Australia expands demand for both cost-efficient reusable systems and selective single-use deployment. Large urban hospitals invest in advanced imaging processors and comprehensive reprocessing suites, while secondary centers prioritize durable scopes and vendor-supported training. Local manufacturing and distribution partnerships improve service coverage and parts availability. Government initiatives to strengthen endoscopy infrastructure, combined with growing pancreatobiliary case loads, support steady procedure growth.Middle East & Africa
Tertiary hospitals in the Gulf and key African metros anchor demand, focusing on infection-control credibility, warranty reliability, and onsite training. Procurement often bundles scopes, processors, AERs, and service into turnkey solutions to streamline setup. Limited reprocessing capacity in some settings catalyzes interest in single-use scopes for select cases, while major centers favor reusable platforms with disposable critical parts. Vendor presence, installer competence, and access to loaners are decisive for uptime.South & Central America
Referral centers in Brazil, Mexico, and the Southern Cone adopt modern reusable platforms with improved elevator mechanics and imaging upgrades, supported by strengthened reprocessing protocols. Budget variability encourages hybrid fleets - reusable scopes for routine volume and single-use units for infection-risk or overflow contexts. Training partnerships and regional service hubs address skill gaps and maintenance turnaround. Institutions prioritize solutions that document cleaning compliance, reduce cancellations, and stabilize procedure throughput across public and private systems.Duodenoscopes Market Segmentation
By Product
- Flexible video duodenoscope
- Flexible non-video duodenoscope
By Application
- Diagnostic
- Treatment
By End-User
- Hospitals
- Outpatient Facilities
Key Market players
Olympus Corporation, PENTAX Medical (HOYA Corp.), Fujifilm Holdings Corporation, Boston Scientific Corporation, Ambu A/S, Karl Storz SE & Co. KG, Cook Medical LLC, EndoFresh Medical Inc., SonoScape Medical Corp., B. Braun Melsungen AGDuodenoscopes Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Duodenoscopes Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Duodenoscopes market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Duodenoscopes market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Duodenoscopes market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Duodenoscopes market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Duodenoscopes market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Duodenoscopes value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Duodenoscopes industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Duodenoscopes Market Report
- Global Duodenoscopes market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Duodenoscopes trade, costs, and supply chains
- Duodenoscopes market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Duodenoscopes market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Duodenoscopes market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Duodenoscopes supply chain analysis
- Duodenoscopes trade analysis, Duodenoscopes market price analysis, and Duodenoscopes supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Duodenoscopes market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Olympus Corporation
- PENTAX Medical (HOYA Corp.)
- Fujifilm Holdings Corporation
- Boston Scientific Corporation
- Ambu A/S
- Karl Storz SE & Co. KG
- Cook Medical LLC
- EndoFresh Medical Inc.
- SonoScape Medical Corp.
- B. Braun Melsungen AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
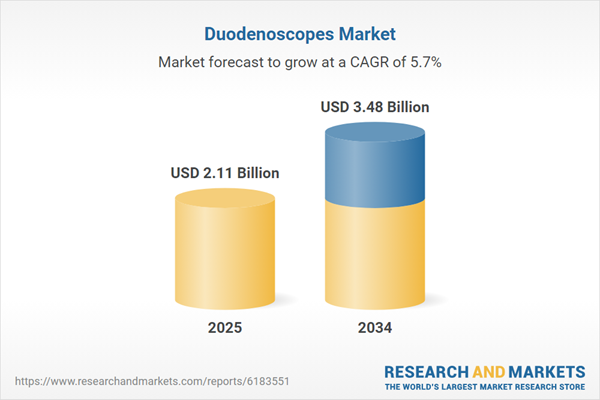
| Estimated Market Value ( USD | $ 2.11 Billion |
| Forecasted Market Value ( USD | $ 3.48 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |