Anti-CD20 Monoclonal Antibodies (MAbs) Market
The anti-CD20 monoclonal antibodies market encompasses agents that deplete B-cells to treat B-cell malignancies and immune-mediated diseases. Core indications include non-Hodgkin lymphoma and chronic lymphocytic leukemia across front-line and relapsed settings, often in combination with chemotherapy, BTK inhibitors, or BCL-2 inhibitors. Autoimmune end-uses span multiple sclerosis (relapsing and primary progressive), rheumatoid arthritis, ANCA-associated vasculitis, pemphigus vulgaris, nephrotic syndromes, and off-label uses managed under specialist protocols. Portfolios include chimeric, humanized, and fully human antibodies with distinct mechanisms (type I vs. type II), Fc-engineering for enhanced ADCC, and subcutaneous (SC) or intravenous (IV) formats; lifecycle strategies add hyaluronidase-enabled SC co-formulations and autoinjectors for home use. Trends emphasize site-of-care shift from infusion centers to home/self-administration in neurology, co-development with targeted small molecules in oncology, and precision dosing guided by minimal residual disease. Drivers include guideline entrenchment in hematology, expanding neurologic labels, payer acceptance of biosimilars that broaden access, and real-world evidence supporting durable disease control with manageable safety. Competitive intensity features originators defending franchises with new formulations and outcomes data, next-generation glycoengineered antibodies, and a robust wave of rituximab biosimilars/biobetters from global and regional players. Headwinds include CAR-T and T-cell engagers encroaching on later-line lymphoma, infection-risk stewardship (hypogammaglobulinemia, HBV reactivation, PML vigilance), vaccine-timing considerations, and pricing pressure from tenders and reference pricing. Vendors balancing efficacy, convenience, pharmacoeconomic value, and safety governance are best placed as care pathways integrate anti-CD20s alongside cellular and targeted therapies.Anti-CD20 Monoclonal Antibodies (MAbs) Market Key Insights
- Dual market backbone: oncology and autoimmunity
- Formulation and site-of-care innovation
- Biosimilars expand access and reset price tiers
- Glycoengineering and type II mechanisms
- Combination therapy is the default in CLL/NHL
- Neurology shifts toward self-management
- Safety governance is a buying criterion
- Real-world evidence shapes value narratives
- Competition from cell and bispecific therapies
- Manufacturing resilience and cold-chain excellence
Anti-CD20 Monoclonal Antibodies (MAbs) Market Reginal Analysis
North America
High specialist density and established infusion infrastructure sustain broad use across oncology and neurology. Payers incentivize biosimilar adoption in hospitals while maintaining access to originators in select settings. Site-of-care steering encourages SC and home administration where safe; robust REMS-like safety protocols and vaccination guidance are standard.Europe
Reference pricing and tendering accelerate biosimilar penetration, especially in inpatient oncology. Neurology uptake grows for relapsing and progressive MS with emphasis on pharmacovigilance and pregnancy planning. Health-technology assessments favor options with strong real-world data and SC convenience; hospital formularies enforce interchangeability frameworks.Asia-Pacific
Diverse access profiles: mature markets adopt next-gen and SC formats, while emerging markets scale rituximab biosimilars via public procurement. Oncology demand rises with expanding diagnostic capacity; neurology growth follows MS center development. Local fill-finish and regional partnerships improve supply and affordability.Middle East & Africa
Tertiary centers in GCC anchor originator and next-gen use; broader regions rely on cost-effective biosimilars through government tenders. Priorities include cold-chain reliability, Arabic/French IFUs, and hepatitis screening infrastructure. Neurology services expand with specialist recruitment and infusion-to-SC transition programs.South & Central America
Public systems and social insurers drive volume via competitive tenders and local biosimilar policies. Private hospitals maintain mixed portfolios to balance cost and continuity of care. Growing MS diagnosis and lymphoma treatment capacity elevate demand; stable logistics and pharmacist-led switching protocols support adherence.Anti-CD20 Monoclonal Antibodies (MAbs) Market Segmentation
By Product
- First Generation Anti-cd20 Monoclonal Antibody
- Second Generation Anti-cd20 Monoclonal Antibody
- Third Generation Anti-cd20 Monoclonal Antibody
By Application
- Oncology
- Neurology
- Immunology
Key Market players
Roche/Genentech, Novartis, TG Therapeutics, Pfizer, Amgen, Sandoz, Celltrion Healthcare, Teva Pharmaceutical Industries, Dr. Reddy’s Laboratories, Shanghai Henlius Biotech, BIOCAD, Intas Pharmaceuticals (Accord Healthcare), Hetero Labs, Zydus Lifesciences, Chugai PharmaceuticalAnti-CD20 Monoclonal Antibodies (MAbs) Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Anti-CD20 Monoclonal Antibodies (MAbs) Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Anti-CD20 Monoclonal Antibodies (MAbs) market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Anti-CD20 Monoclonal Antibodies (MAbs) market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Anti-CD20 Monoclonal Antibodies (MAbs) market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Anti-CD20 Monoclonal Antibodies (MAbs) market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Anti-CD20 Monoclonal Antibodies (MAbs) market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Anti-CD20 Monoclonal Antibodies (MAbs) value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Anti-CD20 Monoclonal Antibodies (MAbs) industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Anti-CD20 Monoclonal Antibodies (MAbs) Market Report
- Global Anti-CD20 Monoclonal Antibodies (MAbs) market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Anti-CD20 Monoclonal Antibodies (MAbs) trade, costs, and supply chains
- Anti-CD20 Monoclonal Antibodies (MAbs) market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Anti-CD20 Monoclonal Antibodies (MAbs) market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Anti-CD20 Monoclonal Antibodies (MAbs) market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Anti-CD20 Monoclonal Antibodies (MAbs) supply chain analysis
- Anti-CD20 Monoclonal Antibodies (MAbs) trade analysis, Anti-CD20 Monoclonal Antibodies (MAbs) market price analysis, and Anti-CD20 Monoclonal Antibodies (MAbs) supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Anti-CD20 Monoclonal Antibodies (MAbs) market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Roche/Genentech
- Novartis
- TG Therapeutics
- Pfizer
- Amgen
- Sandoz
- Celltrion Healthcare
- Teva Pharmaceutical Industries
- Dr. Reddy’s Laboratories
- Shanghai Henlius Biotech
- BIOCAD
- Intas Pharmaceuticals (Accord Healthcare)
- Hetero Labs
- Zydus Lifesciences
- Chugai Pharmaceutical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
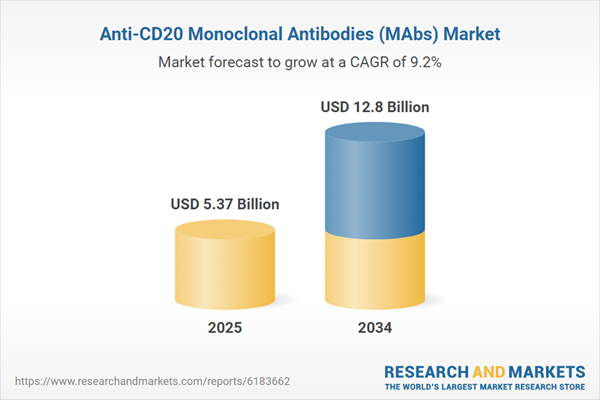
| Estimated Market Value ( USD | $ 5.37 Billion |
| Forecasted Market Value ( USD | $ 12.8 Billion |
| Compound Annual Growth Rate | 9.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |