Osteoarthritis Injectables Market
The Osteoarthritis (OA) Injectables market spans corticosteroids (including extended-release), hyaluronic acid (HA) viscosupplements across single- and multi-shot regimens, platelet-rich plasma (PRP) and autologous conditioned products, as well as emerging biologics (amniotic-derived, MSC-adjacent preparations) and investigational disease-modifying intra-articular candidates. Core end-uses include primary knee OA, hip and shoulder involvement, and earlier interventions in active, overweight, or post-injury populations seeking surgery deferral. Trends emphasize non-opioid pain control, ultrasound-guided precision placement, phenotype-guided care (inflammatory vs mechanical pain), and combination protocols (e.g., HA + PRP; corticosteroid for flare followed by HA). Growth catalysts include aging demographics, rising obesity, higher imaging utilization, and patient preference for outpatient, rapid-recovery procedures. The competitive landscape features specialty pharmaceutical companies, orthobiologics innovators, compounding networks, and integrated orthopedic groups/ASCs that bundle diagnostics, injections, and rehabilitation. Differentiation hinges on durability of analgesia, functional improvement, onset speed, injection burden, and safety (post-injection flare, chondrotoxicity, infection control). Payer dynamics remain pivotal: variability in guideline endorsements, cost-effectiveness assessments, and step-therapy rules influence regimen choice and site-of-care. Execution priorities include robust evidence packages (RCTs and real-world registries), standardized PRP preparation and characterization, cold-chain and viscosity management for HA, and operator training for ultrasound techniques. Challenges persist around mixed guideline positions on HA, heterogeneity of PRP formulations, limited head-to-head data across classes, reimbursement friction in certain indications, and the need for credible disease-modifying signals beyond symptomatic relief.Osteoarthritis Injectables Market Key Insights
- Treatment paradigms are stratifying by OA phenotype and patient goals. Clinics increasingly segment inflammatory phenotypes (effusion/synovitis) toward corticosteroid or biologic-leaning regimens for rapid flare control, while mechanical pain with cartilage wear skews to HA or combination strategies for glide and function. Shared decision-making weighs onset speed, durability, and activity targets. Ultrasound confirmation of effusion and accurate intra-articular placement reduces non-response risk and improves patient-reported outcomes over landmark techniques.
- Hyaluronic acid remains the workhorse where reimbursement aligns - but with shifts. Single-injection, high-molecular-weight and cross-linked HAs reduce visit burden and appeal to active patients; multi-shot series anchor conservative pathways in some systems. Providers emphasize syringe ergonomics, extrusion force, and viscosity stability at room temp. Real-world registries guide selection by age, BMI, baseline WOMAC, and prior steroid exposure, supporting targeted use despite heterogeneous guideline endorsements.
- Extended-release steroids redefine the role of corticosteroids. Microsphere or depot formulations extend analgesia while aiming to limit peak systemic exposure and chondrotoxicity concerns. Positioning centers on acute flare control, bridge-to-rehab, and pre-event activity windows. Protocols cap annual dose frequency and rotate joints to mitigate risks, while ultrasound triage distinguishes synovitis-driven pain from structural stiffness before dosing decisions.
- PRP/orthobiologics gain traction but require standardization. Clinics increasingly disclose platelet concentration, leukocyte profile, and activation method to reduce variability. Evidence is strongest in mild-to-moderate knee OA, with durability advantages in select cohorts; however, payer acceptance lags. Combination regimens (PRP following HA, or staged after steroid flare control) are explored to balance onset and persistence with fewer visits and lower total cost-in-use.
- Ultrasound guidance is now a quality and equity issue, not just a premium add-on. Image-guided placement improves accuracy in obese patients and challenging joints (hip, shoulder), lowering dry taps and procedural pain. Practices standardize probe hygiene, sterile technique, and needle approach angles to shorten table time and reduce post-injection flare. Integration with point-of-care documentation supports audit trails and payer pre-auth.
- Safety and chondroprotection narratives are differentiators. Vendors highlight excipient profiles, endotoxin thresholds, and pH/osmolality ranges that minimize flares. Practices adopt bundles - skin prep, needle choice, aspiration policies - to reduce septic arthritis risk. For biologics, donor-screening rigor, device IFU compliance, and adverse-event registries shape hospital formulary access and corporate tenders.
- Evidence portfolios are moving beyond pain scores. Sponsors add gait analysis, activity trackers, and cartilage thickness mapping to link symptom relief with function. Minimal clinically important difference (MCID) and responder analyses trump mean changes for payer dialog. Head-to-head pragmatics vs usual care in real clinics carry more weight than small RCTs with narrow inclusion criteria.
- Site-of-care economics drive product choice. Ambulatory and office settings prefer single-visit efficacy, low prep time, and minimal cold-chain friction; ASCs value predictable turnover and sterile workflows. Bundled payment pilots and digital scheduling reduce no-shows and compress cycle times. Products with simple prior-auth pathways and clear coding guide adoption even when list prices are higher.
- Combination and sequencing protocols are the next competitive frontier. Staged care - steroid for acute synovitis, followed by HA for function, and PRP for durability - seeks to maximize net benefit while spacing visits. Early data suggest ultrasound-verified effusion control before HA improves adherence and outcomes. Protocolized sequencing, rather than single-class loyalty, gains favor in integrated ortho networks.
- Pipeline shifts from symptomatic relief toward structure-modifying aims. Novel injectables target catabolic cytokines, pain-sensory pathways, and cartilage anabolism; some pair with extended-release carriers or hydrogels for residency. Success will hinge on safety in repeated dosing, MRI/mechanistic biomarkers, and demonstration of surgery deferral - key to unlocking payer enthusiasm and premium pricing.
Osteoarthritis Injectables Market Reginal Analysis
North America
High procedure volumes through orthopedic groups and ASCs, with strong adoption of ultrasound guidance and extended-release steroids. Reimbursement scrutiny shapes HA utilization; private pay expands PRP and combination protocols. Patient portals, e-consent, and remote PROMs support longitudinal outcomes tracking. Competitive focus: durability narratives, streamlined prior-auth, and site-of-care efficiency.Europe
Guideline and HTA heterogeneity drives country-specific pathways: HA is selectively reimbursed; PRP is prevalent in private pay and sports clinics. Strong emphasis on ultrasound standards, infection control, and clinic accreditation. Hospitals and group practices negotiate formulary access on safety dossiers and cost-per-responder metrics; rehabilitation integration is routine.Asia-Pacific
Broad uptake of HA in markets with long clinical familiarity; rapid growth of PRP in private orthopedics and sports medicine. Aging demographics and active populations in Japan, Australia, South Korea, and urban China expand addressable volumes. Ultrasound-guided hip and shoulder injections rise; premium segments value single-visit solutions and minimal downtime.Middle East & Africa
Demand concentrated in GCC private hospitals and sports/rehab centers, with cash-pay HA and PRP leading. Hot-climate logistics require robust cold-chain and clinic SOPs. Medical tourism and elite sports programs drive early adoption of combination protocols; bilingual labeling and clinician training influence product selection.South & Central America
Selective growth via private ortho and pain clinics, with HA as anchor and expanding PRP offerings. Currency volatility and import dynamics favor products with stable supply and clear coding. Training on ultrasound guidance and standardized PRP preparation improves outcomes; partnerships with rehab networks support adherence and repeat cycles.Osteoarthritis Injectables Market Segmentation
By Type
- Hyaluronic Acid Injections
- Corticosteroid Injections
- Platelet-rich Plasma (PRP) Injections
- Placental Tissue Matrix (PTM) Injections
- Acetylsalicylic Acid (ASA) Injections
- Others
By Anatomy
- Knee Osteoarthritis
- Hip Osteoarthritis
- Hand Osteoarthritis
- Others
By End-User
- Hospital Pharmacies
- Retail Pharmacies
- Others
Key Market players
Sanofi, Anika Therapeutics, Fidia Farmaceutici, Seikagaku Corporation, Bioventus, Zimmer Biomet, TRB Chemedica, IBSA Group, Pacira BioSciences, Pfizer, Merck & Co. (MSD), Bristol Myers Squibb, Arthrex, Terumo BCT, Regen LabOsteoarthritis Injectables Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Osteoarthritis Injectables Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Osteoarthritis Injectables market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Osteoarthritis Injectables market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Osteoarthritis Injectables market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Osteoarthritis Injectables market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Osteoarthritis Injectables market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Osteoarthritis Injectables value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Osteoarthritis Injectables industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Osteoarthritis Injectables Market Report
- Global Osteoarthritis Injectables market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Osteoarthritis Injectables trade, costs, and supply chains
- Osteoarthritis Injectables market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Osteoarthritis Injectables market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Osteoarthritis Injectables market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Osteoarthritis Injectables supply chain analysis
- Osteoarthritis Injectables trade analysis, Osteoarthritis Injectables market price analysis, and Osteoarthritis Injectables supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Osteoarthritis Injectables market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Sanofi
- Anika Therapeutics
- Fidia Farmaceutici
- Seikagaku Corporation
- Bioventus
- Zimmer Biomet
- TRB Chemedica
- IBSA Group
- Pacira BioSciences
- Pfizer
- Merck & Co. (MSD)
- Bristol Myers Squibb
- Arthrex
- Terumo BCT
- Regen Lab
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
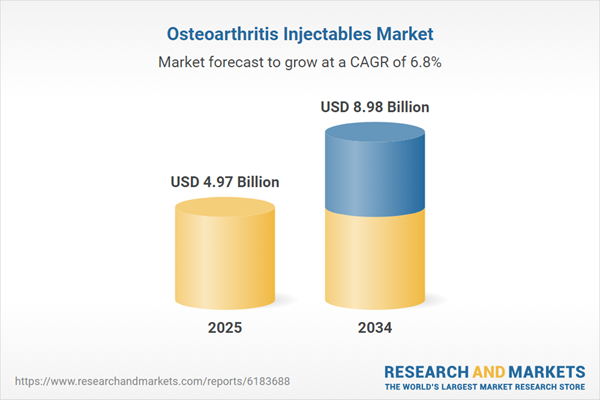
| Estimated Market Value ( USD | $ 4.97 Billion |
| Forecasted Market Value ( USD | $ 8.98 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |