Joint Replacement Devices Market
The Joint Replacement Devices market spans hip, knee, shoulder, elbow, ankle, and small-joint arthroplasty systems - implant constructs, robotics-enabled instruments, patient-specific guides, navigation, and digital care platforms. Top applications are total knee and hip replacements in degenerative osteoarthritis, avascular necrosis, rheumatoid disease, trauma sequelae, and revision surgeries; shoulder (anatomic and reverse) grows with rotator cuff arthropathy, while ankle and small joints expand niche indications. Trends include cementless, porous 3D-printed surfaces for biologic fixation, kinematics-informed designs (medial-pivot, single-radius), cross-linked liners and oxidation-resistant bearings, and intraoperative personalization via robotics, navigation, and alignment philosophies (mechanical, kinematic, restricted). Drivers are aging populations, earlier intervention among active patients, ERAS pathways that reduce length of stay, and hospital/ASC migration through minimally invasive approaches and streamlined trays. Competition centers on comprehensive portfolios, surgeon education ecosystems, and digital tools (pre-op planning, intra-op analytics, remote rehab) that document outcomes. Vendors differentiate with portfolio breadth (primary to complex revision), enabling tech integration, efficient sterilization logistics, and reliable inventory/service. Headwinds include pricing pressure from tenders and bundled payments, scrutiny of implant longevity and rare device events, staffing variability, and revision complexity. Overall, the category is shifting from product-centric procurement to outcomes-based partnerships where implant design, enabling technology, and pathway execution jointly deliver functional recovery, alignment fidelity, and predictable economics.Joint Replacement Devices Market Key Insights
- Enabling tech drives consistency: Robotics, imageless navigation, and patient-specific planning standardize cuts and soft-tissue balance, narrowing outliers and supporting same-day or next-day discharge with fewer reworks.
- Cementless expands beyond hips: Advanced porous and bioactive surfaces accelerate osseointegration in knees and shoulders, appealing to younger, active cohorts and revision risk reduction strategies where bone quality permits.
- Bearing materials underpin longevity: Highly cross-linked polyethylene with antioxidant stabilization and ceramic heads mitigate wear and osteolysis; articulation matching (conforming vs. mobile) tailors kinematics to patient goals.
- Personalized alignment philosophies: Mechanical, kinematic, and restricted kinematic alignment coexist; data-rich planning plus intra-op feedback helps surgeons reconcile alignment targets with ligament balance and gait restoration.
- ASC migration reshapes design: Lighter, tray-reduced instrument sets, single-use disposables for critical steps, and rapid-recovery anesthetic protocols enable efficient ambulatory workflows and lower sterile processing burdens.
- Revision and extremity growth: Complex revisions, reverse shoulder, and total ankle expand as techniques and implants improve; modular augments, sleeves, and cones support bone loss management and fixation in compromised anatomy.
- Digital spine of the episode: Pre-op templating, inventory analytics, and remote rehab capture outcomes and PROMs, enabling value-based contracts and continuous improvement loops across health systems.
- Education ecosystems are moats: Cadaver labs, simulators, and proctoring accelerate adoption of new techniques and robotics; vendors with robust clinical support reduce variability during learning curves.
- Supply assurance matters: Kit accuracy, set readiness, and predictable replenishment reduce case delays; vendors offering vendor-managed inventory and on-site field engineering win share.
- Sustainability and compliance rise: Tray optimization, reusable components, and waste minimization align with hospital ESG goals, while UDI tracking, registries, and post-market surveillance inform iterative design.
Joint Replacement Devices Market Reginal Analysis
North America
Adoption is propelled by ERAS pathways, robotics/navigation penetration, and ASC migration for select primary joints. Health systems prioritize vendors that demonstrate reduced LOS, fewer readmissions, and predictable episode costs. Competitive differentiation hinges on digital planning ecosystems, inventory reliability, and on-site clinical support. Pricing pressure persists under bundled payments, favoring tray-reduced systems and outcomes documentation.Europe
Guideline-driven practice and national registries emphasize implant survivorship, revision rates, and cost-effectiveness. Cementless options gain share in hips and select knees, while reverse shoulder sees strong growth. Procurement via tenders favors comprehensive portfolios with sustainability credentials and robust post-market evidence. Training networks and standardized pathways support consistent outcomes across public hospital trusts.Asia-Pacific
Rising procedure volumes follow urbanization and expanded access to advanced care. Premium centers adopt robotics, patient-specific planning, and cementless knees for younger patients, while value segments prioritize proven implants with efficient instrumentation. Local manufacturing and distributor service footprints influence awards. Surgeon training partnerships and fellowship exchanges accelerate diffusion of MIS and alignment philosophies.Middle East & Africa
Adoption concentrates in tertiary medical hubs and private hospitals, with strong demand for complex revision, reverse shoulder, and navigation-assisted primaries. Procurement emphasizes turnkey solutions - implants, enabling tech, service, and training. Reliability of supply, rapid instrument servicing, and standardized ERAS protocols are pivotal to scale programs across variable skill bases.South & Central America
Private networks in major metros drive growth, complemented by public tenders focused on cost-effective, durable implants. Surgeon preference for familiar systems remains strong; education, proctoring, and service responsiveness sway conversions. Currency and import dynamics heighten attention to TCO, tray optimization, and vendor-managed inventory. Digital rehab and PROMs collection gain traction where connectivity supports remote follow-up.Joint Replacement Devices Market Segmentation
By Product
- Knee Replacement Devices
- Hip Replacement Devices
- Shoulder Replacement Devices
- Ankle Replacement Devices
- Elbow Replacement Devices
- Wrist Replacement Devices
- Bone Graft Substitutes
- Others
By Surgery
- Total Replacement Procedures
- Partial Replacement Procedures
- Revision Replacement Procedures
By Type
- Cemented Fixation
- Cementless Fixation
- Hybrid Fixation
- Reverse Hybrid Fixation
By Technique
- Traditional Surgery
- Minimally Invasive Surgery
- Computer-Assisted Surgery
By End-User
- Hospitals & Surgical Centers
- Ambulatory Care Centers & Trauma Units
- Orthopedic Clinics
Key Market players
Stryker, Zimmer Biomet, DePuy Synthes (Johnson & Johnson MedTech), Smith+Nephew, Enovis (including LimaCorporate/DJO), Exactech, MicroPort Orthopedics, Medacta International, B. Braun Aesculap, Waldemar Link, Corin Group, Kyocera Medical, United Orthopedic Corporation, AK Medical Group, Groupe LépineJoint Replacement Devices Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Joint Replacement Devices Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Joint Replacement Devices market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Joint Replacement Devices market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Joint Replacement Devices market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Joint Replacement Devices market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Joint Replacement Devices market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Joint Replacement Devices value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Joint Replacement Devices industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Joint Replacement Devices Market Report
- Global Joint Replacement Devices market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Joint Replacement Devices trade, costs, and supply chains
- Joint Replacement Devices market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Joint Replacement Devices market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Joint Replacement Devices market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Joint Replacement Devices supply chain analysis
- Joint Replacement Devices trade analysis, Joint Replacement Devices market price analysis, and Joint Replacement Devices supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Joint Replacement Devices market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Stryker
- Zimmer Biomet
- DePuy Synthes (Johnson & Johnson MedTech)
- Smith+Nephew
- Enovis (including LimaCorporate/DJO)
- Exactech
- MicroPort Orthopedics
- Medacta International
- B. Braun Aesculap
- Waldemar Link
- Corin Group
- Kyocera Medical
- United Orthopedic Corporation
- AK Medical Group
- Groupe Lépine
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
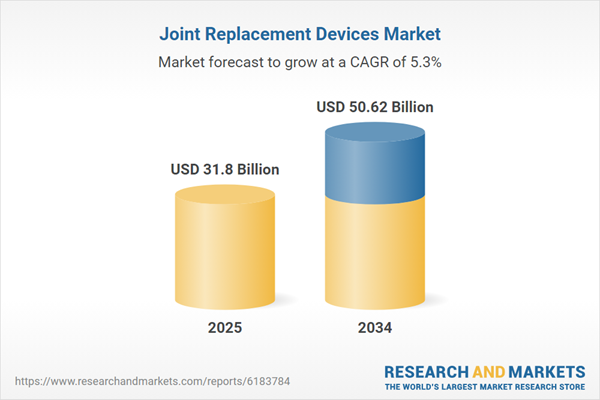
| Estimated Market Value ( USD | $ 31.8 Billion |
| Forecasted Market Value ( USD | $ 50.62 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |