Nonmydriatic Handheld Fundus Camera Market
Nonmydriatic handheld fundus cameras are portable retinal imaging devices that capture diagnostic-grade images through an undilated pupil, enabling rapid screening at the point of care. They are increasingly deployed beyond ophthalmology - into primary care clinics, diabetes centers, retail pharmacies, community screening camps, mobile vans, and teleophthalmology hubs - where ease of use, short acquisition time, and minimal patient preparation are critical. The top end-uses today revolve around diabetic retinopathy screening in primary care and endocrinology settings, opportunistic detection of glaucoma suspects via optic nerve head imaging, and documentation of macular/vascular changes in optometry and community programs. Product and workflow innovation is brisk: modern units emphasize improved optics/sensors for small-pupil performance, on-device image quality guidance, cloud connectivity, and seamless interoperability with EMR/EHR and tele-reading platforms. A visible trend is tighter integration with decision-support and autonomous/assistive AI to streamline triage and reduce reading bottlenecks. Market drivers include the rising burden of diabetes and aging populations, the shift to value-based care and community screening mandates, and the expansion of retail and virtual care models. The competitive landscape mixes diversified ophthalmic imaging brands with agile specialists and smartphone-based entrants, competing on image quality, reliability, total cost of ownership, and service coverage. While tabletop systems still dominate advanced diagnostics, handheld nonmydriatic devices win on portability, access, and throughput in non-specialist settings. Key challenges remain around operator training, consistent image quality in real-world environments, cybersecurity and privacy safeguards, and procurement models that balance up-front device cost with software, service, and AI subscriptions.Nonmydriatic Handheld Fundus Camera Market Key Insights
- Point-of-care first adoption. The strongest demand signal comes from primary care and diabetes pathways seeking fast retinal screening without dilation. Handheld form factor, battery operation, and simple fixation targets allow imaging in exam rooms and retail clinics, reducing referral leakage and improving screening adherence. Programs value short learning curves for medical assistants, durable housings for frequent transport, and immediate image verification to minimize retakes and keep patient flow steady.
- AI-enabled workflows are moving on-device. Vendors increasingly pair cameras with assistive or autonomous AI for diabetic retinopathy triage, quality grading, and routing. This compresses screening-to-decision time, supports staff in low-resource settings, and can standardize outputs for population programs. Buyers compare not just AI accuracy claims but also audit trails, explainability options, update cadence, and the ability to decouple hardware from analytics partners as needs evolve.
- Smartphone-based vs. dedicated handheld trade-offs. Smartphone attachments lower entry cost and ease upgrades, while dedicated handhelds offer ruggedization, optimized optics, and validated image consistency. Procurement teams assess image sharpness across small pupils, flare control, and low-light performance, alongside accessory ecosystems (eye cups, chin rests) that stabilize capture in high-throughput campaigns and pediatric or elderly populations.
- Small-pupil performance is a visible differentiator. Advances in sensors, illumination, and algorithms are improving success rates in ambient light without pharmacologic dilation. Vendors compete on usable field of view, peripheral vessel clarity, and consistent macula/optic disc capture. Real-world programs emphasize first-pass success to protect throughput and patient experience, with some adopting ambient-light shields and standardized positioning protocols.
- Interoperability and data stewardship drive enterprise wins. Health systems and retail chains prioritize DICOM/FHIR compatibility, single sign-on, role-based access, and automated routing to reading centers. Secure cloud storage, encryption, and regional data residency options are now baseline requirements. Fleet management (remote diagnostics, firmware/AI updates, usage analytics) is becoming part of the value proposition for multi-site deployments.
- Reimbursement and care-model alignment matter. Coverage for remote retinal imaging and evolving codes for AI-supported assessments encourage in-clinic screening by non-specialists. Value-based contracts and quality metrics elevate screening completion targets, making lightweight cameras attractive tools for closing care gaps. Retail health and employer clinics add incremental demand as they extend chronic-disease services.
- Total cost of ownership (TCO) over sticker price. Buyers look beyond device list price to include software licenses, AI fees, cloud storage, training, and multi-year service. Durable designs, swappable batteries, and simplified calibration reduce downtime. Vendors offering tiered software bundles, predictable subscriptions, and evidence of low retake rates often show superior lifecycle economics.
- Hybrid channel strategies expand reach. Established ophthalmic brands leverage hospital/optometry channels, while specialists scale via primary-care distributors, pharmacy partnerships, and NGO frameworks. In emerging markets, local assembly, service networks, and financing (leasing, pay-per-use, or subscription) can be decisive, particularly for public tenders and community eye-health programs.
- Use-case broadening beyond diabetes. While DR screening dominates, handheld nonmydriatic cameras increasingly support documentation of AMD changes, hypertensive retinopathy, and optic nerve head monitoring for glaucoma risk. Integration with tele-glaucoma and tele-retina pathways helps expand utilization, strengthening the business case for multi-department procurement across health systems.
- Quality assurance and governance are purchase criteria. Programs seek vendor support for standardized training, proficiency tracking, and image-quality audits. Procurement asks for illumination safety documentation, cybersecurity hardening guides, and post-market surveillance processes. Vendors that provide implementation playbooks, clinical protocols, and outcome dashboards shorten time-to-value and de-risk scale-up.
Nonmydriatic Handheld Fundus Camera Market Reginal Analysis
North America
Demand is propelled by large diabetes populations, quality metrics in value-based contracts, and the expansion of screening in primary care and retail clinics. AI-enabled workflows are gaining traction where they reduce reading burden and accelerate referrals. Procurement emphasizes interoperability with major EHR platforms, HIPAA-grade security, and fleet analytics for multi-state networks. Training toolkits for medical assistants and pharmacy staff, along with robust service coverage, are key differentiators for enterprise rollouts.Europe
Community and primary-care screening initiatives sustain steady uptake, with hospital outpatient and optometry settings complementing demand. Buyers require CE-compliant devices, strong privacy governance aligned with regional regulations, and seamless integration with national or regional tele-ophthalmology pathways. Tender-based procurement favors vendors with proven reliability, multilingual UIs, and established service partners. Programs often pair handheld devices with centralized reading centers to standardize grading and quality oversight.Asia-Pacific
Large, diverse markets adopt handheld cameras to extend screening into community health centers, company clinics, and mobile outreach. Price sensitivity and high throughput needs support interest in smartphone-based solutions, while tertiary hospitals and private chains lean toward ruggedized dedicated handhelds. Governments and NGOs run periodic screening drives, creating spikes in demand for fleets, accessories, and rapid training. Local manufacturing, distributor depth, and financing options meaningfully influence vendor selection.Middle East & Africa
Access expansion is the dominant theme, with handheld nonmydriatic devices enabling screening outside tertiary eye hospitals. Public-private partnerships, donor-funded pilots, and mobile eye camps favor portable, battery-reliable systems with intuitive workflows. Training, remote support, and simple serviceability are prioritized to maintain uptime across dispersed geographies. Data-sovereignty requirements and offline-first workflows can shape platform choices in select countries.South & Central America
Public health programs targeting diabetic eye disease and cardiovascular risk drive steady adoption in primary care and community clinics. Buyers value devices that balance affordability with dependable image quality and straightforward cloud connectivity to regional reading hubs. Local distribution, Spanish/Portuguese interfaces, and responsive after-sales service are critical. Emerging local innovators and regional assembly can improve availability and TCO, especially for government tenders and NGO deployments.Nonmydriatic Handheld Fundus Camera Market Segmentation
By Type
- Optical System
- Image Acquisition System
By Application
- Fundus Imaging
- Fluorescein Angiography
Key Market players
ZEISS, Optomed, Remidio, Volk Optical, Welch Allyn (Baxter), Topcon Healthcare, MediWorks, NIDEK, Phelcom Technologies, D-EYE, JEDMED (MiiS), Ezer, oDocs Eye Care, Forus Health, Epipole.Nonmydriatic Handheld Fundus Camera Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Nonmydriatic Handheld Fundus Camera Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Nonmydriatic Handheld Fundus Camera market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Nonmydriatic Handheld Fundus Camera market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Nonmydriatic Handheld Fundus Camera market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Nonmydriatic Handheld Fundus Camera market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Nonmydriatic Handheld Fundus Camera market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Nonmydriatic Handheld Fundus Camera value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Nonmydriatic Handheld Fundus Camera industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Nonmydriatic Handheld Fundus Camera Market Report
- Global Nonmydriatic Handheld Fundus Camera market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Nonmydriatic Handheld Fundus Camera trade, costs, and supply chains
- Nonmydriatic Handheld Fundus Camera market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Nonmydriatic Handheld Fundus Camera market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Nonmydriatic Handheld Fundus Camera market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Nonmydriatic Handheld Fundus Camera supply chain analysis
- Nonmydriatic Handheld Fundus Camera trade analysis, Nonmydriatic Handheld Fundus Camera market price analysis, and Nonmydriatic Handheld Fundus Camera supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Nonmydriatic Handheld Fundus Camera market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- ZEISS
- Optomed
- Remidio
- Volk Optical
- Welch Allyn (Baxter)
- Topcon Healthcare
- MediWorks
- NIDEK
- Phelcom Technologies
- D-EYE
- JEDMED (MiiS)
- Ezer
- oDocs Eye Care
- Forus Health
- Epipole.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
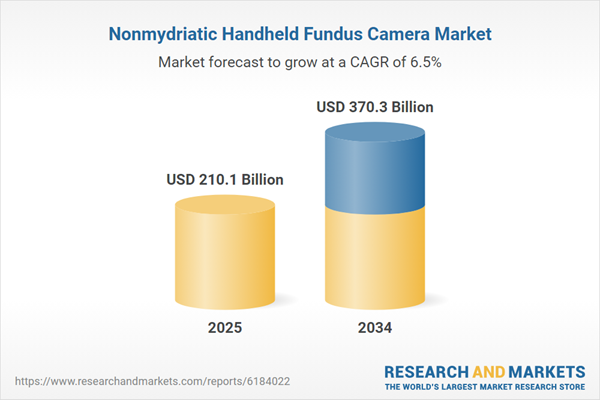
| Estimated Market Value ( USD | $ 210.1 Billion |
| Forecasted Market Value ( USD | $ 370.3 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |