Disposable Surgical Devices Market
The Disposable Surgical Devices Market comprises single-use instruments and consumables that enable sterile, reliable, and efficient procedures across general surgery, orthopedics, cardiovascular, gynecology/urology, ENT, neuro, ophthalmic, and ambulatory settings. Portfolios span scalpels and safety blades, trocars, laparoscopic hand instruments (grasper, dissector, scissors), staplers and reloads, energy/ligation devices with sterile tips, suction-irrigation sets, electrosurgical pencils, catheters and guidewires, biopsy and specialty needles, wound closure (sutures, endoscopic clips), drapes/gowns, and procedure-specific packs. Key trends center on infection prevention and workflow certainty: factory-sterile products reduce reprocessing variability, support fast room turns, and standardize performance for robotics and image-guided surgery. Design shifts favor lighter ergonomics, tactile feedback, and color-coded cues; packaging integrates clear IFUs, tray organization, and sustainability features. Hospitals and ASCs lean into custom procedure packs that pre-bundle critical SKUs and reduce pick errors. Drivers include rising surgical volumes, ASC migration, heightened SSI vigilance, staff shortages pushing simpler workflows, and payer pressure on predictable episode costs. Competitive dynamics span diversified medtech leaders, focused single-use innovators, private label, and OEM/ODM suppliers; differentiation rests on cut/seal performance, staple formation quality, tip sharpness, reliable ergonomics, and service models that optimize inventory and waste. Constraints include price sensitivity versus reusables, environmental scrutiny of single-use plastics, supply-chain resilience for sterilization capacity and materials, and training to ensure proper device disposal and sharps safety. Overall, disposables are evolving from commodity adjuncts to engineered, pack-integrated solutions that deliver consistent sterility, efficiency, and clinical outcomes across diverse sites of care.Disposable Surgical Devices Market Key Insights
- Infection control as a mandate: Factory-sterile, single-use instruments mitigate reprocessing variability and biofilm risk - supporting SSI reduction goals and enabling rapid OR/ASC turnover without capacity bottlenecks.
- Pack integration boosts efficiency: Custom procedure packs and case carts reduce SKU touches, pick errors, and set-up time; standardized contents simplify training and enable reproducible outcomes across teams and sites.
- Performance parity vs. reusable sets: Advances in metallurgy, edge geometry, and polymer bearings deliver precise cutting, grip strength, and rotary smoothness; consistent “out-of-box” performance curbs mid-case switch-outs.
- Stapling and energy reliability: Staple line integrity, tissue compression sensing, and reload availability are decisive; disposable energy tips and pencil wands ensure consistent seal quality and safety.
- Ergonomics & human factors: Low-force actuation, anti-slip grips, ambidextrous layouts, and color-coded sizing reduce fatigue and errors, especially in long MIS cases or teaching hospitals.
- Data-ready logistics: GS1 labeling, UDI, and tray-level barcodes streamline charge capture, recall readiness, and consumption analytics; auto-replenishment lowers stockouts and waste.
- ASC migration favors single-use: Short turnovers, lean staffing, and limited sterile processing capacity make disposables the default for many specialties, with clear cost-per-case accounting.
- Sustainability pressure, real solutions: Material light-weighting, mono-material trays, device-only recycling/take-back pilots, and minimized pouch headspace reduce waste without compromising sterility.
- Supply resilience matters: Dual sterilization pathways (EtO/low-temp), diversified component sourcing, and regional DCs protect elective case schedules from disruptions.
- Training & safety culture: Sharps-injury prevention (retractable/safety designs), intuitive IFUs, and standardized disposal protocols protect staff and support accreditation audits.
Disposable Surgical Devices Market Reginal Analysis
North America
ASC expansion and value-based purchasing favor single-use with predictable cost-per-case and rapid room turns. Health systems prioritize UDI traceability, robust vendor service, and pack standardization across networks. Sustainability programs focus on lightweight packaging and selective recycling pilots without jeopardizing sterility or throughput.Europe
Procurement emphasizes documented clinical performance, eco-design, and compliance with packaging and waste directives. Public tenders reward standardized packs, sharps safety features, and supply continuity. Reprocessing alternatives are assessed rigorously, yet single-use remains preferred for complex MIS and infection-sensitive workflows.Asia-Pacific
Rising surgical access and private-hospital growth drive volume; cost-effective, reliable disposables and procedure packs support fast build-outs. Japan/Korea value precision and compact packaging; Australia/New Zealand stress sustainability; China/India scale with locally manufactured SKUs plus premium imports for tertiary centers.Middle East & Africa
Flagship hospitals and burgeoning private sectors adopt premium single-use for infection control and staffing efficiency. Procurement favors turnkey packs, multilingual IFUs, and dependable sterilization/supply logistics. Broader markets seek value-engineered options with strong distributor support and training.South & Central America
Economic variability elevates the importance of clear cost-in-use and flexible contracting. Private networks and PPPs adopt standardized packs for general and laparoscopic surgery. Reliable local distribution, UDI/traceability, and sharps safety features are key to awards and sustained adoption.Disposable Surgical Devices Market Segmentation
By Product
- Surgical sutures & staplers
- Handheld surgical devices
- Electrosurgical devices
By Application
- Neurosurgery
- Plastic & reconstructive surgery
- Wound closure
- Obstetrics & gynecology
- Cardiovascular
- Orthopedic
- General surgery
- Others
Key Market players
Johnson & Johnson (Ethicon), Medtronic, 3M Health Care, Becton, Dickinson and Company (BD), B. Braun Melsungen AG, Smith & Nephew, Stryker, Teleflex, CONMED, Integra LifeSciences, Mölnlycke Health Care, Cardinal Health, Halyard Health (Owens & Minor), Medline Industries, Applied MedicalDisposable Surgical Devices Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Disposable Surgical Devices Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Disposable Surgical Devices market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Disposable Surgical Devices market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Disposable Surgical Devices market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Disposable Surgical Devices market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Disposable Surgical Devices market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Disposable Surgical Devices value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Disposable Surgical Devices industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Disposable Surgical Devices Market Report
- Global Disposable Surgical Devices market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Disposable Surgical Devices trade, costs, and supply chains
- Disposable Surgical Devices market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Disposable Surgical Devices market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Disposable Surgical Devices market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Disposable Surgical Devices supply chain analysis
- Disposable Surgical Devices trade analysis, Disposable Surgical Devices market price analysis, and Disposable Surgical Devices supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Disposable Surgical Devices market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Johnson & Johnson (Ethicon)
- Medtronic
- 3M Health Care
- Becton
- Dickinson and Company (BD)
- B. Braun Melsungen AG
- Smith & Nephew
- Stryker
- Teleflex
- CONMED
- Integra LifeSciences
- Mölnlycke Health Care
- Cardinal Health
- Halyard Health (Owens & Minor)
- Medline Industries
- Applied Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
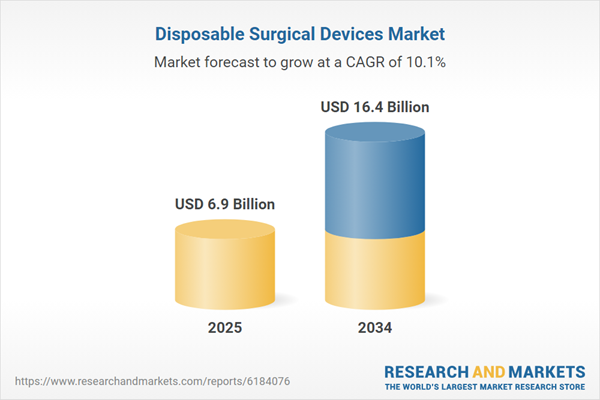
| Estimated Market Value ( USD | $ 6.9 Billion |
| Forecasted Market Value ( USD | $ 16.4 Billion |
| Compound Annual Growth Rate | 10.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |