Biologics Manufacturing Market
The Biologics Manufacturing Market spans monoclonal antibodies and Fc-engineered formats, recombinant proteins and enzymes, vaccines (viral, protein subunit, conjugate), advanced modalities such as cell and gene therapies, viral vectors, plasmid DNA, oncolytic platforms, and nucleic-acid therapeutics including mRNA/LNP. End-uses cut across oncology, immunology, infectious disease, rare diseases, and emerging metabolic and neurology indications. The operating model blends in-house networks of large pharma with global CDMO capacity, increasingly configured as multi-modal campuses that co-locate drug substance, fill-finish, and analytics. Structural trends include single-use intensification in upstream, modular and ballroom facilities, high-capacity chromatography and membrane separations, real-time analytics (PAT), and digital twins that compress tech transfer and scale-up. Drivers are robust pipelines, lifecycle management and indications expansion for launched biologics, accelerated pathways for high-need diseases, and regionalization of supply for resilience. Counterweights include talent scarcity, raw-material lead times (resins, filters, lipids, bags), regulatory expectations on data integrity and comparability, and mounting pressure to decarbonize energy-intensive operations. Competitive dynamics feature originator biologics expanding capacity for launch readiness, biosimilar entrants optimizing cost-per-gram and throughput, and CDMOs racing to secure sticky, end-to-end programs from cell line to commercial. As platform processes for antibodies mature and next-gen modalities diversify, winners are those that harmonize process platforms with flexible facilities, orchestrate digital QA/QC at scale, de-risk supply with qualified dual sources, and demonstrate credible CMC execution from first-in-human through lifecycle changes without disrupting supply continuity.Biologics Manufacturing Market Key Insights
- Modality mix is diversifying beyond mAbs
- Single-use intensification vs. stainless scale economies
- Continuous and intensified processing moves from pilot to plant
- Fill-finish is the rate-limiting step more often than DS
- Viral vectors, plasmids, and LNPs require specialized ecosystems
- Supply chain resilience is a board-level KPI
- Quality, data integrity, and regulatory readiness define credibility
- Pharma 4.0 and advanced analytics compress timelines
- Human capital is the scarcest bioprocess input
- Cost, sustainability, and footprint now share the same dashboard
Biologics Manufacturing Market Reginal Analysis
North America
Biologics leadership is anchored by deep pipelines, large mAb installed base, and a vibrant CDMO sector. Multi-modal expansions prioritize DS/DP co-location, high-speed PFS and vial isolators, and intensified upstream retrofits in existing shells. Policy incentives and procurement encourage regional supply resilience. Talent competition is intense; companies differentiate with academies and digital workcells. CGT clusters scale vector and cell-processing capacity with stringent segregation and release testing.Europe
A quality- and regulation-focused ecosystem with strong originator, biosimilar, and vaccine footprints. Investments emphasize continuous processing, Annex 1-ready aseptic suites, and sustainability programs integrated into utilities and waste streams. Regionalization strategies add redundancy for critical medicines. Public-private partnerships support advanced platforms (LCM antibodies, ADCs, mRNA). CDMOs win with end-to-end offerings and proven tech-transfer speed across multi-country networks.Asia-Pacific
Rapid capacity build-out spans antibodies, vaccines, and nucleic-acid therapeutics, with competitive cost structures and fast project cycles. Government programs back domestic supply and export ambitions; local innovators move from biosimilar to novel pipelines. Single-use greenfield plants dominate, with growing stainless for scale mAbs. Fill-finish additions chase domestic launch readiness. Talent scaling, raw-material localization, and global compliance are near-term focus areas.Middle East & Africa
Select hubs invest in vaccine and biologics self-reliance, often via tech-transfer alliances and modular plants. Priorities include fill-finish capability, cold chain, and QC labs that meet global standards. Workforce development and supplier ecosystems are building from low bases. Regional procurement favors reliable DP for public health programs. Long-term plans target upstream DS capacity and broader modality coverage as skills deepen.South & Central America
National institutes and private partners expand vaccine and biologics capabilities to enhance regional autonomy. Upgrades focus on aseptic DP, QC modernization, and selective DS suites for priority therapeutics. Policy support and tender visibility underpin investment cases. Talent pipelines and supplier logistics (resins, filters, single-use) remain constraints. Collaborations with global CDMOs accelerate compliance and tech transfer for time-sensitive programs.Biologics Manufacturing Market Segmentation
By Type
- Biologics
- Biosimilars
By Application
- Outsourced
- In-house
Key Market players
Lonza, Samsung Biologics, WuXi Biologics, Fujifilm Diosynth Biotechnologies, Boehringer Ingelheim BioXcellence, Thermo Fisher Scientific (Patheon), Catalent, AGC Biologics, Rentschler Biopharma, AbbVie, Amgen, Roche (Genentech), Novartis, Sanofi, Eli Lilly, Bristol Myers Squibb, AstraZeneca, Pfizer, Johnson & Johnson (Janssen), Biocon BiologicsBiologics Manufacturing Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Biologics Manufacturing Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Biologics Manufacturing market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Biologics Manufacturing market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Biologics Manufacturing market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Biologics Manufacturing market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Biologics Manufacturing market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Biologics Manufacturing value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Biologics Manufacturing industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Biologics Manufacturing Market Report
- Global Biologics Manufacturing market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Biologics Manufacturing trade, costs, and supply chains
- Biologics Manufacturing market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Biologics Manufacturing market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Biologics Manufacturing market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Biologics Manufacturing supply chain analysis
- Biologics Manufacturing trade analysis, Biologics Manufacturing market price analysis, and Biologics Manufacturing supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Biologics Manufacturing market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Lonza
- Samsung Biologics
- WuXi Biologics
- Fujifilm Diosynth Biotechnologies
- Boehringer Ingelheim BioXcellence
- Thermo Fisher Scientific (Patheon)
- Catalent
- AGC Biologics
- Rentschler Biopharma
- AbbVie
- Amgen
- Roche (Genentech)
- Novartis
- Sanofi
- Eli Lilly
- Bristol Myers Squibb
- AstraZeneca
- Pfizer
- Johnson & Johnson (Janssen)
- Biocon Biologics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
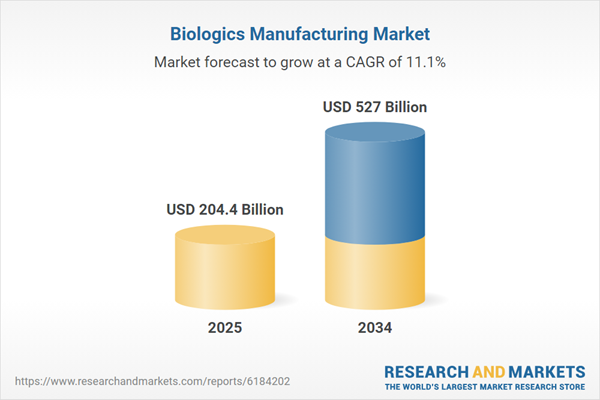
| Estimated Market Value ( USD | $ 204.4 Billion |
| Forecasted Market Value ( USD | $ 527 Billion |
| Compound Annual Growth Rate | 11.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |