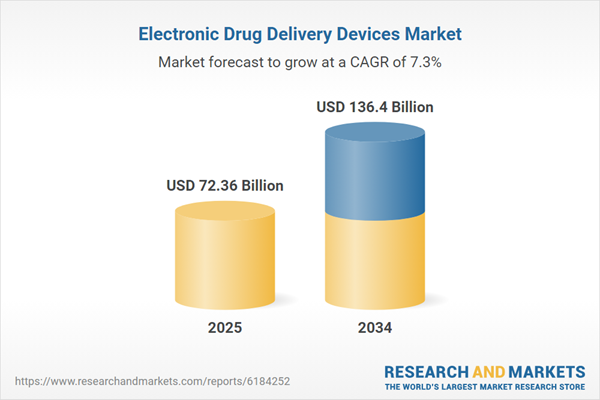
Electronic Drug Delivery Devices Market
The Electronic Drug Delivery Devices Market encompasses connected and electromechanical systems that measure, control, and document dosing across chronic and acute therapies. Core platforms include smart autoinjectors and pens for biologics, on-body injectors and wearables for large-volume/viscous drugs, connected inhalers and nebulizers for respiratory care, electronic infusion pumps and ambulatory PCA, connected insulin delivery (pens, pumps, patch pumps), and smart ophthalmic/dermal applicators. End-uses span immunology, diabetes, oncology/supportive care, migraine, cardiovascular and lipid disorders, respiratory diseases, women’s health, and rare disease home infusion. Trends emphasize patient-centric design - quiet motors, needle-concealment, and guided UI - paired with connectivity (BLE/NFC), dose logging, reminders, and interoperability with EHRs and disease apps. Drivers include biologics growth, shift to home and alternate sites of care, payer focus on adherence and outcomes, and workforce constraints in clinics. The competitive landscape blends large medtech and pharma-device alliances, digital therapeutics partners, sensor and firmware specialists, and CDMOs with electromechanical and combination-product expertise. Differentiation rests on reliability (force/occlusion sensing, fail-safes), human factors and usability data, cybersecurity and data privacy, supply resilience for chips and batteries, and total cost-to-serve under value-based contracts. Challenges include regulatory complexity for combination products, connectivity fragmentation, battery and e-waste stewardship, and equitable access beyond high-income settings. Overall, electronic delivery is shifting from “device add-on” to integral therapy platforms - combining accurate dosing, digital coaching, and real-world data to improve persistence, reduce adverse events, and lower total cost of care.Electronic Drug Delivery Devices Market Key Insights
- Home-first delivery: On-body injectors, connected pens, and ambulatory pumps support care migration from hospital to home, reducing chair time and enabling flexible scheduling without compromising safety.
- Data-enabled adherence: Automated dose capture, reminders, and pattern insights reduce missed doses; payer and provider portals leverage real-world data for risk stratification and targeted outreach.
- Human factors as a moat: Intuitive loading, tactile/visual/audible cues, and fail-safes (auto-needle retraction, lockouts) drive confidence; usability evidence underpins labeling and payer adoption.
- Large-volume biologics readiness: Wearable injectors, motor/drive tuning, and heat management enable subcutaneous delivery of high-viscosity, high-volume formulations - expanding home eligibility for specialty drugs.
- Interoperability & standards: Open APIs, FHIR-based data exchange, and Bluetooth profiles streamline integration with diabetes apps, respiratory platforms, and provider dashboards, reducing tech fatigue.
- Cybersecurity & privacy: Secure bootloaders, encrypted comms, and lifecycle patching protect PHI and therapy integrity; threat modeling and SBOM transparency are increasingly tender requirements.
- Power and sustainability: Rechargeable or extended-life batteries, energy-aware firmware, and take-back programs address e-waste; design-for-serviceability extends device lifespan across refills.
- Manufacturing resilience: Dual-sourcing chips/sensors, automated assembly, and design-for-test reduce bottlenecks; robust suppliers for cannulas, adhesives, and primary containers stabilize supply.
- Regulatory sophistication: Combination-product pathways, software validation, and post-market surveillance maturity (complaints, MDRs) differentiate partners; companion apps follow SaMD expectations.
- Equity & usability at scale: Multi-language UI, low-vision and dexterity accommodations, and offline modes broaden access; price-tiered connectivity (NFC-only SKUs) expands global reach.
Electronic Drug Delivery Devices Market Reginal Analysis
North America
Adoption is propelled by specialty biologics, diabetes technology leadership, and reimbursement models that reward adherence and remote monitoring. Health systems and payers value integrated portals, proven usability, and strong cybersecurity. Home infusion and alternate-site clinics expand on-body injector and ambulatory pump use. Supply assurance for chips/batteries and robust service networks weigh heavily in vendor selection.Europe
Strong regulatory and data-privacy frameworks steer emphasis on clinical evidence, human factors documentation, and secure data handling. National tenders favor interoperable, standards-based connectivity and e-waste stewardship. Hospital-to-home pathways and pharmacy-led services support connected pens and wearables. Sustainability metrics and device reusability influence procurement across the EU and UK.Asia-Pacific
Rapid growth in chronic disease management, digital health familiarity, and government telehealth initiatives drive connected inhalers, diabetes devices, and smart injectors. Japan/Korea prioritize precision and compact design; China scales through digital ecosystems and local manufacturing; India and Southeast Asia value cost-sensitive, durable devices with offline usability. Partnerships with hospitals and e-pharmacies accelerate adoption.Middle East & Africa
GCC markets lead with premium connected devices in specialty centers and home-care programs, prioritizing reliability, data security, and service responsiveness. Broader MEA adoption focuses on ruggedness, long battery life, and multilingual interfaces. Public-private initiatives and distributor-led education support growth, with emphasis on training and simplified supply chains.South & Central America
Payers and ministries explore connected delivery to improve adherence in diabetes, respiratory, and immunology programs. Devices must tolerate heat/humidity and variable connectivity; NFC-enabled logging and SMS workflows gain traction. Local distributor networks, affordability tiers, and serviceable designs influence tenders. Pharmacist-led coaching and bundled therapy kits aid persistence.Electronic Drug Delivery Devices Market Segmentation
By Product
- Smart Infusion Pumps
- Smart Metered Dose Inhalers
- Smart Transdermal Patches
- Others
By Application
- Diabetes
- Respiratory Diseases
- Oncology
- Cardiology
- Others
Key Market players
Medtronic, Insulet Corporation, Tandem Diabetes Care, Ypsomed, SHL Medical, West Pharmaceutical Services, Phillips-Medisize (Molex), Aptar Pharma, Teva Pharmaceutical Industries, ResMed (Propeller Health), Aerogen, PARI Pharma, Enable Injections, Gerresheimer, Becton, Dickinson and Company (BD)Electronic Drug Delivery Devices Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Electronic Drug Delivery Devices Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Electronic Drug Delivery Devices market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Electronic Drug Delivery Devices market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Electronic Drug Delivery Devices market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Electronic Drug Delivery Devices market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Electronic Drug Delivery Devices market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Electronic Drug Delivery Devices value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Electronic Drug Delivery Devices industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Electronic Drug Delivery Devices Market Report
- Global Electronic Drug Delivery Devices market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Electronic Drug Delivery Devices trade, costs, and supply chains
- Electronic Drug Delivery Devices market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Electronic Drug Delivery Devices market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Electronic Drug Delivery Devices market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Electronic Drug Delivery Devices supply chain analysis
- Electronic Drug Delivery Devices trade analysis, Electronic Drug Delivery Devices market price analysis, and Electronic Drug Delivery Devices supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Electronic Drug Delivery Devices market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Medtronic
- Insulet Corporation
- Tandem Diabetes Care
- Ypsomed
- SHL Medical
- West Pharmaceutical Services
- Phillips-Medisize (Molex)
- Aptar Pharma
- Teva Pharmaceutical Industries
- ResMed (Propeller Health)
- Aerogen
- PARI Pharma
- Enable Injections
- Gerresheimer
- Becton
- Dickinson and Company (BD)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 72.36 Billion |
| Forecasted Market Value ( USD | $ 136.4 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |