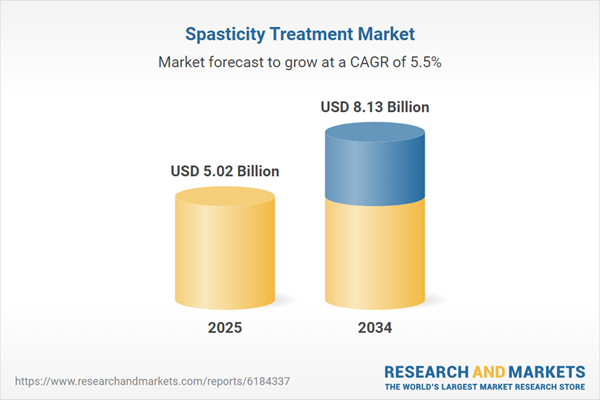
Spasticity Treatment Market
The Spasticity Treatment market spans pharmacologic therapies, interventional procedures, and rehabilitative modalities aimed at managing velocity-dependent muscle hypertonia following neurological injury or disease. Core end-uses include post-stroke rehabilitation, cerebral palsy (pediatric and adult), multiple sclerosis, spinal cord injury, and traumatic brain injury; post-operative orthopedic and neuro-oncology cases form smaller but important niches. Care is increasingly multimodal: oral antispasmodics (baclofen, tizanidine, dantrolene) remain first-line in diffuse presentations, while botulinum toxin injections dominate for focal and multi-focal patterns using EMG/ultrasound guidance and individualized dosing algorithms. For generalized or refractory cases, intrathecal baclofen (ITB) pumps, chemical neurolysis (phenol/alcohol), selective peripheral neurotomy, and, in select centers, neuromodulation techniques complement intensive physical and occupational therapy, orthoses, and digital home programs. Trends shaping demand include earlier intervention along post-acute pathways, broader use of image-guided chemodenervation, longer-acting toxin formulations and biosimilars, device miniaturization with smarter dosing analytics, and remote monitoring that links clinics, therapists, and caregivers. Driving factors comprise rising stroke survival, aging populations, improved diagnosis of pediatric neuromotor disorders, and payer recognition of functional outcomes that lower falls, contractures, and caregiver burden. Competition is two-speed: established biopharma leaders and device innovators defend premium brands through evidence and services, while generics and value-based contracts expand access in cost-sensitive systems. Providers differentiate through multidisciplinary clinics, protocolized outcome tracking, and day-surgery injection pathways that reduce wait times. Over the forecast horizon, innovation will emphasize durability, precision, and safety - target-specific toxins, refill-optimized pumps, and digital phenotyping - to deliver measurable gains in mobility and quality of life.Spasticity Treatment Market Key Insights
- Clinical segmentation guides modality choice. Focal spasticity (e.g., wrist/finger flexors, ankle equinus) favors botulinum toxin with guidance imaging; diffuse patterns drive oral agents or ITB pumps. Multidisciplinary algorithms prioritize functional goals - gait speed, hygiene, pain relief - over tone reduction alone, improving patient-reported outcomes and adherence.
- Botulinum toxin remains cornerstone for focal disease. Broader adoption of ultrasound/EMG guidance, dilution strategies, and muscle selection matrices increases effect consistency and reduces adverse events. Longer-acting and room-temperature-stable toxins plus biosimilar entries are expanding practice options and shaping formulary decisions and training.
- Oral antispasmodics evolve toward tolerability. Clinicians balance efficacy with sedation, hypotension, and hepatotoxicity risks, using nighttime dosing, slow titration, and combination regimens. Personalized approaches - pharmacogenomic awareness, deprescribing in older adults, and switch strategies - seek sustained function with fewer systemic side effects.
- Intrathecal baclofen for refractory generalized cases. MRI-conditional pumps, programmable dosing schedules, and telemetry aid precise delivery with lower systemic exposure. Programs emphasize peri-implant screening, refill logistics, and withdrawal risk protocols, with expanding use in complex pediatric spasticity and dystonia-spasticity overlap.
- Image-guided interventions raise precision. Ultrasound, EMG, and, in specialized settings, electrical stimulation mapping reduce mis-injection and improve dose efficiency. Procedure standardization - needle approach, reconstitution, and post-injection stretching - supports reproducible outcomes across networks and satellite clinics.
- Rehabilitation and digital layers amplify gains. Task-specific therapy, orthoses, FES, and home-based telerehab platforms extend benefits beyond clinic visits. Wearable sensors, patient-reported outcome apps, and video-guided exercises create feedback loops that tune dosing intervals and therapy intensity.
- Safety and risk management remain central. Programs strengthen checklists for toxin storage and reconstitution, monitor for antibody-mediated non-response, and maintain emergency pathways for ITB withdrawal. Education for caregivers and school-based therapists reduces complications and unplanned care.
- Value-based care and documentation. Payers increasingly require goal attainment scaling, spasticity-related pain metrics, and functional endpoints to justify continued therapy. Bundled pathways - assessment, injection, therapy, orthotics - streamline authorizations and shorten time to intervention.
- Pediatric and transition-of-care focus. Early, goal-directed management in cerebral palsy emphasizes growth-aware dosing, contracture prevention, and orthotic integration. Structured transition from pediatric to adult services prevents therapy gaps, aligning with education and vocational goals.
- Innovation horizon. Next-generation toxins targeting specific SNARE interactions, programmable pumps with longer refill intervals, less neurolytic chemical options, and selective neurotomy refinements aim for durability and fewer adverse effects. Data-driven scheduling and AI-assisted muscle targeting emerge alongside registries that benchmark outcomes.
Spasticity Treatment Market Reginal Analysis
North America
Care pathways are anchored in comprehensive neurorehabilitation networks with strong adoption of botulinum toxin, image guidance, and ITB for refractory cases. Payer policies emphasize documented functional gains, pushing providers to use standardized scales and digital outcomes. Ambulatory surgery centers and office-based injection suites support efficient throughput, while multidisciplinary clinics coordinate therapy, orthotics, and caregiver education. Large academic systems lead training and registries, accelerating best-practice diffusion to community settings.Europe
Well-established neurorehabilitation programs and guideline-driven practice support broad access to focal injections and advanced pumps, with rigorous outcomes tracking. Public procurement and tendering influence brand selection, while specialist centers expand ultrasound-guided protocols and day-case pathways. Pediatric cerebral palsy services are integrated with physiotherapy and orthotics, and cross-border reference networks share complex case expertise. Workforce training and standardized care bundles underpin consistent quality across regions.Asia-Pacific
Expanding stroke services, rising MS awareness, and improving pediatric neurology unlock demand for focal toxin therapy and structured rehab. Access varies widely: urban tertiary hospitals adopt ultrasound-guided injections and ITB; secondary centers prioritize oral therapies and stepped-care models. Government programs and private insurers increasingly recognize functional outcomes, supporting early intervention. Local training initiatives and partnerships with device and pharma companies build capacity beyond major metros.Middle East & Africa
Centers of excellence in major cities offer comprehensive spasticity management, while peripheral access remains uneven. Investment in rehabilitation hospitals and training scholarships is improving availability of guided injections and pump programs. Care models often rely on caregiver education and home programs, with teleconsults linking remote patients to specialists. Procurement priorities include product stability, supply continuity, and service support for pumps and guidance equipment.South & Central America
Urban neurology and rehab hubs lead adoption of botulinum toxin, with growing interest in ultrasound guidance and protocolized dosing. Public systems and private payors gradually expand coverage where functional outcomes demonstrate reduced complications and caregiver burden. Access to ITB is concentrated in reference hospitals, emphasizing patient selection and refill infrastructure. Partnerships with physiotherapy schools and community programs improve adherence and continuity post-injection.Spasticity Treatment Market Segmentation
By Type
- Drug therapy
- Physical therapy
- Surgical treatment
By End-User
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Diagnostic Centers
- Others
Key Market players
Amcor plc, Silgan Holdings, Inc., Ardagh Group, Berry Plastics Corp., Plastipak Holdings, Inc., Sonoco Products Company, Graham Packaging Company, Inc., Weener Plastics, Ball Corporation, Tetra PakSpasticity Treatment Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Spasticity Treatment Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Spasticity Treatment market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Spasticity Treatment market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Spasticity Treatment market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Spasticity Treatment market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Spasticity Treatment market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Spasticity Treatment value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Spasticity Treatment industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Spasticity Treatment Market Report
- Global Spasticity Treatment market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Spasticity Treatment trade, costs, and supply chains
- Spasticity Treatment market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Spasticity Treatment market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Spasticity Treatment market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Spasticity Treatment supply chain analysis
- Spasticity Treatment trade analysis, Spasticity Treatment market price analysis, and Spasticity Treatment supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Spasticity Treatment market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Amcor PLC
- Silgan Holdings Inc.
- Ardagh Group
- Berry Plastics Corp.
- Plastipak Holdings Inc.
- Sonoco Products Company
- Graham Packaging Company Inc.
- Weener Plastics
- Ball Corporation
- Tetra Pak
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 5.02 Billion |
| Forecasted Market Value ( USD | $ 8.13 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |