Endomyocardial Biopsy Market
The Endomyocardial Biopsy (EMB) market comprises devices, consumables, imaging support, and pathology services used to sample myocardial tissue for histology, immunohistochemistry, and molecular testing. Core end-uses include surveillance of cardiac transplant rejection, etiologic workup of suspected myocarditis, characterization of infiltrative and genetic cardiomyopathies, and investigation of unexplained heart failure or arrhythmias when results alter management. Clinical practice is shifting toward protocolized pathways: fluoroscopy remains the staple, while echocardiography and intracardiac echo broaden access in labs without hybrid suites; electro-anatomic mapping assists targeted left-sided sampling in select cases. On the device side, demand favors single-use bioptomes, lower-profile sheaths, hemostasis valves, and torqueable catheters that improve reach and reduce complications, alongside adjuncts such as pressure monitoring and ultrasound-guided venous access kits. In pathology, digital workflows, AI-assisted image analysis, viral PCR, gene expression profiling, and emerging spatial assays strengthen diagnostic yield and standardization. Growth drivers include stable transplant volumes with intensified early surveillance, heightened myocarditis recognition, precision-medicine programs linking genotype-phenotype, and expanding advanced heart-failure networks. Headwinds include procedure risk (perforation, tamponade), variable operator training, reimbursement scrutiny, and the expanding role of cardiac MRI and PET, which can triage patients away from biopsy in specific scenarios. The competitive landscape features specialized cardiovascular device manufacturers, imaging and navigation vendors, and reference pathology providers forming integrated value propositions around safety, turnaround time, and decision-impact. Over the forecast horizon, adoption will favor safer access, targeted sampling, and data-rich pathology readouts that translate tissue into actionable therapy choices.Endomyocardial Biopsy Market Key Insights
- Transplant surveillance anchors demand. Standardized timelines for early and intermediate post-transplant monitoring sustain procedure volumes; centers differentiate via low-complication access protocols, rapid pathology turnaround, and digital scheduling to minimize readmissions and delays.
- Imaging advances elevate precision. Fluoroscopy plus echo guidance reduces perforation risk and improves sample adequacy; in complex cardiomyopathies, mapping-guided left-ventricular biopsies increase diagnostic yield when right-sided samples are non-representative.
- Device design focuses on safety and control. Smaller French sizes, improved jaw geometry, and enhanced torque transmission support consistent bites with fewer passes. Single-use bioptomes address sterility assurance and logistics, while atraumatic sheaths and closure devices streamline recovery.
- Pathology goes molecular and digital. Beyond hematoxylin-eosin and immunostains, labs deploy viral PCR, immunophenotyping, and gene expression panels to classify rejection and myocarditis subtypes. Whole-slide imaging enables remote reads, QA programs, and AI pre-screening for workload smoothing.
- Myocarditis awareness widens indications. Post-viral and immune-mediated entities drive targeted biopsies when imaging is equivocal or therapy hinges on histology. Institutions formalize multidisciplinary boards to align biopsy timing with immunosuppression decisions.
- Complication mitigation is a differentiator. Ultrasound-guided venous access, real-time pressure checks, and standardized bite counts lower adverse events. Simulation training and proctorship pathways help expand operator pools without compromising outcomes.
- Reimbursement and evidence expectations rise. Payers increasingly request documentation of diagnostic impact; programs respond with pathway metrics - adequacy rates, turnaround times, and treatment changes - demonstrating value beyond the procedural fee.
- Pediatrics and congenital cohorts need specialization. Dedicated tools and protocols for smaller anatomies, plus anesthesia and echo support, enable safe sampling in pediatric transplants and myocarditis, with central pathology review enhancing consistency.
- Alternatives reshape utilization, not relevance. Cardiac MRI and PET reduce unnecessary biopsies but also triage toward higher-yield cases; EMB remains decisive when tissue diagnosis changes immunotherapy, antiviral strategies, or graft management.
- Integration across the care continuum. Vendors bundle devices, imaging, and training; labs pair diagnostics with consultative reports and virtual tumor-board-style reviews. Data feedback loops inform access choice, sample targeting, and follow-up cadence.
Endomyocardial Biopsy Market Reginal Analysis
North America
High transplant and advanced heart-failure center density underpins steady EMB demand, with strong uptake of single-use bioptomes, echo-guided access, and digital pathology. Hospital systems emphasize complication reduction bundles and rapid, actionable reports linking histology to therapy adjustments. Academic networks lead mapping-guided LV biopsies in complex cardiomyopathies, while ambulatory and hybrid labs expand capacity. Reimbursement oversight encourages documentation of clinical impact and standardized pathways.Europe
Guideline-driven practice supports selective but rigorous EMB use: transplant surveillance, biopsy-proven myocarditis, and infiltrative disease confirmation. Procurement favors validated single-use devices and structured reprocessing where reusables persist. University hospitals advance molecular panels and centralized digital reads to harmonize interpretations across regions. Training consortia and simulation programs address operator variability, while day-case protocols and ultrasound-guided venous access improve patient flow.Asia-Pacific
Expanding tertiary cardiac care in China, India, Japan, South Korea, and Australia increases access to EMB, particularly in transplant hubs and metropolitan heart-failure programs. Investments prioritize fluoroscopy/echo integration, reliable disposable supply, and staff upskilling. Imaging often triages first, with biopsy reserved for management-critical decisions. Regional reference labs build capacity for molecular assays and telepathology to support secondary cities and satellite centers.Middle East & Africa
Growth concentrates in major urban transplant and advanced cardiology centers, where turnkey catheter-lab solutions and vendor-supported training accelerate capability. Emphasis is on safety - ultrasound-guided access, standardized post-procedure monitoring, and rapid escalation pathways. Limited local pathology capacity in some markets drives partnerships with centralized labs for molecular testing and second opinions via digital platforms. Procurement values device reliability and responsive service.South & Central America
Referral centers in Brazil, Mexico, Argentina, and Chile anchor demand, focusing on transplant follow-up and selected myocarditis workups. Programs invest in single-use device availability, echo-assisted techniques, and structured complication-avoidance checklists. Regional collaborations expand digital pathology coverage and turnaround, while public-private partnerships support training and equipment refresh. Imaging remains a strong gatekeeper, reserving EMB for cases where tissue diagnosis directs therapy.Endomyocardial Biopsy Market Segmentation
By Product
- Forceps
- Accessories
By Tip
- Maxi-curved
- Straight
- Pre-curved
- Others
By End-User
- Hospitals
- Ambulatory Surgical Centers
- Others
Key Market players
Fonterra Co-operative Group, FrieslandCampina Ingredients, Morinaga Milk Industry Co., Ltd., MILEI GmbH, Synlait Milk Ltd., Westland Milk Products, Tatua Co-operative Dairy Company, Bega Bionutrients (Tatura Milk Industries), Beston Global Food Company, Glanbia Nutritionals, Armor Protéines (Savencia), Ingredia SA, Saputo Dairy Australia, Milk Specialties Global, Hilmar Cheese CompanyEndomyocardial Biopsy Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Endomyocardial Biopsy Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Endomyocardial Biopsy market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Endomyocardial Biopsy market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Endomyocardial Biopsy market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Endomyocardial Biopsy market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Endomyocardial Biopsy market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Endomyocardial Biopsy value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Endomyocardial Biopsy industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Endomyocardial Biopsy Market Report
- Global Endomyocardial Biopsy market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Endomyocardial Biopsy trade, costs, and supply chains
- Endomyocardial Biopsy market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Endomyocardial Biopsy market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Endomyocardial Biopsy market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Endomyocardial Biopsy supply chain analysis
- Endomyocardial Biopsy trade analysis, Endomyocardial Biopsy market price analysis, and Endomyocardial Biopsy supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Endomyocardial Biopsy market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Fonterra Co-operative Group
- FrieslandCampina Ingredients
- Morinaga Milk Industry Co. Ltd.
- MILEI GmbH
- Synlait Milk Ltd.
- Westland Milk Products
- Tatua Co-operative Dairy Company
- Bega Bionutrients (Tatura Milk Industries)
- Beston Global Food Company
- Glanbia Nutritionals
- Armor Protéines (Savencia)
- Ingredia SA
- Saputo Dairy Australia
- Milk Specialties Global
- Hilmar Cheese Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
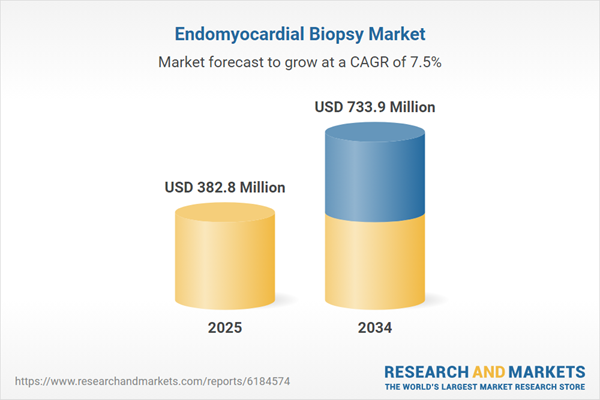
| Estimated Market Value ( USD | $ 382.8 Million |
| Forecasted Market Value ( USD | $ 733.9 Million |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |