Blood Typing Market
The Blood Typing Market encompasses reagents, antisera, gel cards/columns, microplates, lateral-flow cassettes, slide/tube systems, automated analyzers, barcode-ready consumables, and middleware that verify ABO/RhD and extended antigens (Kell, Duffy, Kidd, MNS) across hospitals, blood centers, reference labs, and point-of-care settings. Growth is propelled by transfusion safety mandates, surgical and oncology volumes, trauma preparedness, and prenatal/neo testing - alongside broader screening in donor programs and decentralized care. Vendors differentiate on automation throughput, on-board QC, interpretability of weak D/partial D, and integration with LIS, inventory management, and hemovigilance systems. Workflow priorities include closed, traceable processes from sample collection to crossmatch; consolidation toward gel/column agglutination and solid-phase platforms for reproducibility; and rapid, training-light methods for EDs and ORs. Molecular genotyping complements serology in complex cases, multi-transfused patients, and ethnically diverse populations, enabling phenotype prediction when antisera are limited. Headwinds include staffing shortages, reagent supply variability, and standardization gaps across satellite sites; tailwinds include automation-as-a-service, connectivity, and quality dashboards that reduce repeat testing and near-miss events. Winning offerings pair robust serology with reflex genotyping, turnkey middleware, and service SLAs that keep labs compliant, efficient, and survey-ready.Blood Typing Market Key Insights
- Automation as risk reducer: Benchtop and high-throughput analyzers standardize incubation, centrifugation, and readout, cutting operator variability and turnaround time; on-board QC and positive ID lower clerical error - the top transfusion hazard.
- Gel/column dominance for routine work: Column agglutination delivers clean endpoints and image-assisted grading that supports remote verification; standardized cards simplify training across multi-site systems while preserving sensitivity to weak reactions.
- Rapid methods where minutes matter: Card and cassette formats with visual readouts support trauma bays and ORs; pairing with type-O emergency protocols and confirmatory lab workflows balances speed with safety.
- Weak D/partial D clarity prevents over/under prophylaxis: Algorithms that integrate RhD variant detection reduce unnecessary RhIG in obstetrics and avert alloimmunization in transfusion-dependent patients; middleware guides reflex testing rules.
- Extended typing and phenotype matching cut reactions: Kell, Kidd, Duffy, and MNS screening - augmented by molecular panels - lowers delayed hemolytic risk in oncology, SCD, and thalassemia populations and stabilizes inventory planning.
- Molecular genotyping fills serology gaps: DNA assays resolve recent transfusion interference, autoantibodies, or rare antigen status; labs leverage send-outs or integrated platforms with curated variant databases and reporting templates.
- Connectivity and governance matter: LIS/middleware integration enforces dual verification, delta checks, lot tracking, and audit trails; dashboards surface QC trends, instrument uptime, and operator competency for inspections.
- POC without compromise: ED/ambulance pilots require controls for lot integrity, ambient stability, and operator training; programs succeed when POC results are reconciled automatically with core lab records.
- Supply assurance beats unit price: Dual-sourced antisera, validated alternates, and buffer stock strategies protect trauma readiness; vendor change-control and parallel validation plans prevent downtime during lot transitions.
- Education and competency are the moat: Simulation modules, image libraries of edge cases, and documented challenge sets keep teams survey-ready; vendor field support and proficiency testing underpin sustained performance.
Blood Typing Market Reginal Analysis
North America
Consolidated health systems favor automation, LIS-integrated middleware, and reflex genotyping for complex oncology and SCD cohorts. Trauma networks adopt rapid cards with strict reconciliation workflows. Compliance focus (hemovigilance, operator competency) and strong service SLAs shape supplier selection; inventory analytics support phenotype-matched strategies across multi-hospital networks.Europe
Guideline-driven transfusion services emphasize standardized gel/column platforms, comprehensive QC, and cross-border proficiency schemes. Public tenders value interoperability, reagent traceability, and sustainability in consumables. Molecular typing is embedded for multi-transfused patients, with centralized reference labs supporting satellite hospitals through connected reporting.Asia-Pacific
Large donor programs and expanding surgical capacity drive demand from high-throughput automated systems to cost-efficient manual cards. Urban centers pilot molecular panels for thalassemia/SCD pockets and rare antibodies. Local manufacturing and distributor service depth are decisive; training and multilingual IFUs support rapid scale across diverse sites.Middle East & Africa
Flagship hospitals and national blood services prioritize robust, easy-to-train gel platforms and reliable reagent supply for trauma and maternal care. Bilingual interfaces, heat-tolerant logistics, and remote support are critical. Reference partnerships enable send-out genotyping for complex cases while local labs standardize serology with middleware safeguards.South & Central America
Public-private blood networks expand automation to improve TAT and traceability; affordability drives hybrid models - manual in smaller sites, automated in hubs. Regional reference centers provide extended antigen panels and genotyping. Vendor success hinges on training, inventory resilience, and clear validation toolkits that streamline accreditation across varied hospital tiers.Blood Typing Market Segmentation
By Products & Services
- Instruments
- Reagents & Consumables
- Services
By Test
- Antibody Screening
- HLA Typing
- Cross-matching Tests
- ABO blood Tests
- Others
By Technique
- PCR based and Microarray based
- Assay based techniques
- Massively Parallel Sequencing
- Others
By Application
- Blood transfusions
- Organ transplants
- Diagnostic testing
By End-User
- Hospitals & clinics
- Diagnostic laboratories
- Blood banks
- Others
Key Market players
Bio-Rad Laboratories, QuidelOrtho (Ortho Clinical Diagnostics), Grifols, Immucor (Werfen), Quotient Limited, DIAGAST, BAG Diagnostics (BAG Healthcare), Lorne Laboratories, Sanquin Reagents, Haemokinesis, Hemo Bioscience, Fortress Diagnostics, Tulip Diagnostics (Revvity), Medion Grifols Diagnostics, Rapid Labs Ltd.Blood Typing Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Blood Typing Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Blood Typing market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Blood Typing market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Blood Typing market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Blood Typing market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Blood Typing market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Blood Typing value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Blood Typing industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Blood Typing Market Report
- Global Blood Typing market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Blood Typing trade, costs, and supply chains
- Blood Typing market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Blood Typing market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Blood Typing market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Blood Typing supply chain analysis
- Blood Typing trade analysis, Blood Typing market price analysis, and Blood Typing supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Blood Typing market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Bio-Rad Laboratories
- QuidelOrtho (Ortho Clinical Diagnostics)
- Grifols
- Immucor (Werfen)
- Quotient Limited
- DIAGAST
- BAG Diagnostics (BAG Healthcare)
- Lorne Laboratories
- Sanquin Reagents
- Haemokinesis
- Hemo Bioscience
- Fortress Diagnostics
- Tulip Diagnostics (Revvity)
- Medion Grifols Diagnostics
- Rapid Labs Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
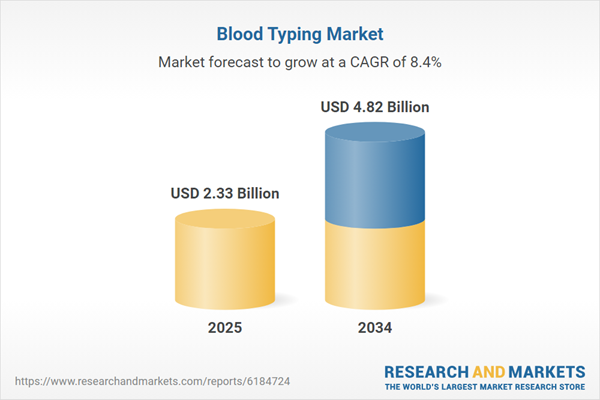
| Estimated Market Value ( USD | $ 2.33 Billion |
| Forecasted Market Value ( USD | $ 4.82 Billion |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |