Spinal Stenosis Implant Market
The Spinal Stenosis Implant market covers devices used to decompress and stabilize the lumbar and cervical spine, including interspinous/interlaminar spacers, posterior fixation (pedicle screws/rods), interbody fusion cages (PEEK, porous titanium, expandable), dynamic stabilization systems, motion-preserving options, and adjunctive biologics/annular closure. Top applications span degenerative lumbar spinal stenosis with neurogenic claudication, cervical spondylotic stenosis/radiculopathy, and revision of failed decompressions. Current trends emphasize minimally invasive and outpatient pathways - microdecompression with interlaminar stabilization, percutaneous pedicle fixation, endoscopic decompression, and lateral/oblique interbody approaches - paired with navigation, robotics, and intraoperative imaging for accuracy and smaller footprints. Growth is propelled by aging populations, earlier diagnosis, patient preference for faster recovery, and payer acceptance of carefully selected MIS procedures with proven functional gains. Competitive dynamics pit diversified spine majors against focused innovators; differentiation turns on implant ergonomics, radiolucency and osseointegration (3D-printed lattices), controlled expansion, sagittal correction capability, and instrument set efficiency. Companies compete through enabling technologies (robotics, navigation, neuromonitoring), sterilization logistics, and surgeon education. Headwinds include scrutiny of spacer-only solutions without robust evidence, variability in reimbursement across care settings, learning curves for advanced MIS/endoscopic techniques, and the need to balance motion preservation with durability. Overall, the category is moving from “hardware-heavy” open surgery to integrated, data-guided MIS ecosystems that aim to deliver reliable decompression, stable alignment, and earlier ambulation with lower perioperative risk.Spinal Stenosis Implant Market Key Insights
- Procedure mix shifts to MIS/outpatient: Microdecompression plus targeted stabilization, lateral/oblique interbodies, and percutaneous fixation migrate cases to ASCs. Vendors that shorten OR time and length of stay gain favor with both surgeons and payers.
- Evidence-first positioning: Payers and societies prioritize implants backed by long-term reoperation and function data; spacer-only indications narrow, while interlaminar stabilization as a decompression adjunct grows where outcomes are durable.
- Enabling tech is a force multiplier: Navigation, robotics, smart drills, and intraoperative CT/fluoro improve accuracy, reduce radiation to staff, and enable smaller incisions - supporting consistent results across varying surgeon volumes.
- Interbody innovation continues: Expandable and lordotic cages with porous titanium surfaces target better endplate contact and fusion biology; radiolucent PEEK with surface treatments seeks imaging clarity plus integration.
- Sagittal alignment matters: Even in stenosis, restoring segmental lordosis correlates with pain and function. Systems that balance decompression with alignment correction see broader adoption.
- Biologics and closure adjuncts: Osteobiologics (DBM, cellular allografts) and annular closure devices reduce pseudarthrosis and reherniation risk in select patients, supporting value narratives when bundled with implants.
- Endoscopic decompression rises: Ultra-MIS endoscopic techniques expand for uniportal/biportal lumbar stenosis; companies offering dedicated access kits and hemostasis tools unlock new surgeon cohorts.
- Economics drive design: Slimmed instrument trays, reprocessable ancillaries, and standardized sets lower sterilization costs and turnover time - important for ASC economics and hospital supply chains.
- Patient selection and care pathways: Prehab, frailty scoring, and opioid-sparing ERAS protocols are now linked to implant decisions; platforms that integrate into pathway analytics help sites hit quality metrics.
- Regulatory and post-market vigilance: Real-world registries, UDI-linked outcomes, and complaint trending shape labeling and training. Vendors with strong surveillance and education reduce variability and litigation exposure.
Spinal Stenosis Implant Market Reginal Analysis
North America
Adoption is anchored by MIS decompression with adjunct stabilization and rapid recovery protocols in hospital-outpatient departments and ASCs. Health systems prioritize implants compatible with navigation/robotics and streamlined trays to cut sterile processing load. Reimbursement favors procedures with proven functional endpoints; scrutiny persists for stand-alone spacers. Surgeon education, rep coverage, and reliable instrument readiness are decisive award factors.Europe
Guideline-driven practice and HTA review emphasize long-term evidence, cost-effectiveness, and complication avoidance. Hospitals favor systems that enable precise decompression and controlled alignment restoration with minimal blood loss. Country-level procurement weighs reusability, tray reduction, and sustainability claims. Endoscopic decompression and interlaminar stabilization gain share in select markets where skill hubs and training networks exist.Asia-Pacific
Large untreated populations and rapid hospital modernization drive demand across open and MIS approaches. Premium urban centers adopt navigation/robotics and expandable interbodies, while secondary markets prioritize robust, cost-efficient fixation and PEEK cages. Training partnerships and visiting-surgeon programs accelerate technique diffusion. Local manufacturing and distributor service footprints influence tender outcomes and aftersales reliability.Middle East & Africa
Tertiary hospitals and medical cities lead adoption, focusing on MIS decompression, percutaneous fixation, and image-guided workflows for complex cases. Procurement favors turnkey packages - implants, instruments, imaging/navigation support, and training. Reliability of supply, rapid instrumentation service, and standardized education modules are key due to variable staff experience and case volumes across regions.South & Central America
Demand concentrates in private hospital networks and academic centers in major metros. Buyers emphasize robust implants with predictable instrumentation, postoperative outcomes that shorten stay, and strong local service. Currency swings and import logistics elevate interest in tray-efficient systems and vendor-managed inventory. Training, proctoring, and outcomes documentation support broader payer acceptance of MIS pathways.Spinal Stenosis Implant Market Segmentation
By Product
- Interspinous Spacer Devices
- Pedicle Screw Based Stabilization Systems
By Material
- Metallic
- Biomaterial
By Surgical Procedure
- Decompression Surgery
- Stabilization Surgery
By End-User
- Hospitals
- Ambulatory Surgical Centers
- Specialty Orthopaedic Clinics
Key Market players
Boston Scientific, Medtronic, DePuy Synthes, Stryker, Zimmer Biomet, Globus Medical, Orthofix, Xtant Medical, Spinal Simplicity, Premia Spine, B. Braun Aesculap, Spine Wave, NuVasive, Aurora Spine, Ulrich MedicalSpinal Stenosis Implant Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Spinal Stenosis Implant Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Spinal Stenosis Implant market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Spinal Stenosis Implant market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Spinal Stenosis Implant market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Spinal Stenosis Implant market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Spinal Stenosis Implant market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Spinal Stenosis Implant value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Spinal Stenosis Implant industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Spinal Stenosis Implant Market Report
- Global Spinal Stenosis Implant market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Spinal Stenosis Implant trade, costs, and supply chains
- Spinal Stenosis Implant market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Spinal Stenosis Implant market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Spinal Stenosis Implant market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Spinal Stenosis Implant supply chain analysis
- Spinal Stenosis Implant trade analysis, Spinal Stenosis Implant market price analysis, and Spinal Stenosis Implant supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Spinal Stenosis Implant market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Boston Scientific
- Medtronic
- DePuy Synthes
- Stryker
- Zimmer Biomet
- Globus Medical
- Orthofix
- Xtant Medical
- Spinal Simplicity
- Premia Spine
- B. Braun Aesculap
- Spine Wave
- NuVasive
- Aurora Spine
- Ulrich Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
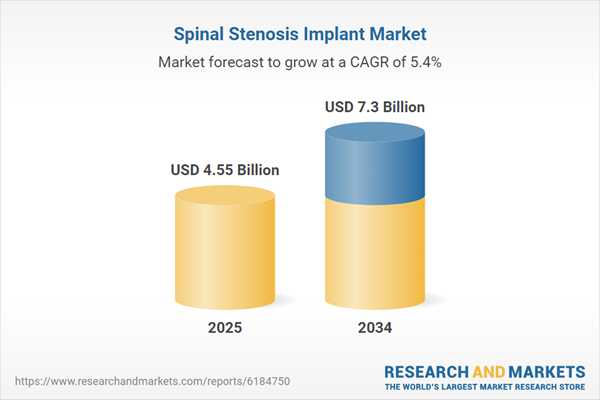
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 4.55 Billion |
| Forecasted Market Value ( USD | $ 7.3 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |