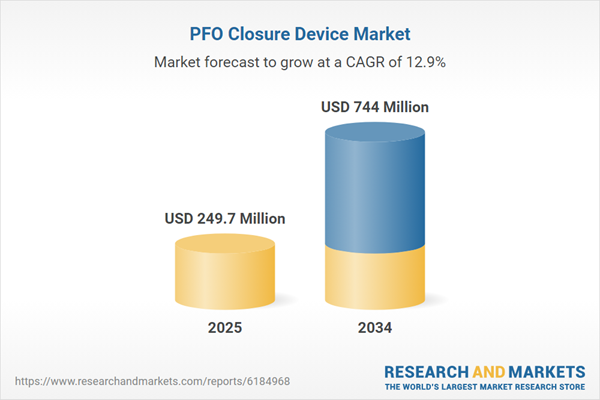
PFO Closure Device Market
The PFO Closure Device Market is evolving from a narrowly defined interventional niche into a core pillar of structural heart therapy, shaped by maturing evidence, standardized care pathways, and closer post-market scrutiny. Clinical demand is centered on secondary stroke prevention in carefully selected patients with cryptogenic stroke, significant right-to-left shunt, or atrial septal aneurysm; adjacent use cases such as migraine with aura and decompression illness remain exploratory and payer-dependent. Hospitals and ambulatory cath labs prioritize systems that simplify transseptal access, deliver reproducible seal, and minimize residual shunt, with low thrombogenicity and device-related arrhythmia as critical safety endpoints. Manufacturers differentiate through device architecture (double-disc vs. conformable frames), atraumatic anchors, nitinol mesh density, bioresorbable or heparin-bonded fabrics, and recapturability for precise placement. Workflow innovation focuses on sheath profiles, steerability, and compatibility with ICE or 3D echo to reduce procedure time, anesthesia needs, and radiation. Procurement is influenced by total episode economics - device price, procedural efficiency, post-closure antithrombotic regimens, and readmission risk - under tighter value-analysis oversight. Competition is intensifying as incumbents defend brand equity with long-horizon safety data while challengers pursue niche anatomies and next-gen materials. Regulatory and reimbursement environments emphasize patient selection algorithms, shared decision-making, and real-world evidence registries tracking stroke recurrence, atrial fibrillation, and device erosion. Training, proctoring, and center-of-excellence models are expanding to lift consistency across community programs. Overall, the category is transitioning toward outcomes-anchored, image-guided solutions that deliver durable closure with low complication rates, positioning PFO devices as a validated option within multidisciplinary stroke prevention strategies. Adoption is broadening across geographies.PFO Closure Device Market Key Insights
- Evidence-led adoption. Growing alignment between neurologists and interventional cardiologists is translating into clearer selection pathways, with emphasis on shunt size, anatomical risk markers, and shared decision-making to optimize net clinical benefit and reduce overtreatment.
- Device design matters. Low-profile, conformable frames with atraumatic anchors and controlled radial force improve apposition and reduce erosion risk, while enhanced fabric technologies target rapid endothelialization and lower residual shunt.
- Imaging-first workflows. Wider use of intracardiac echo and 3D TEE enables conscious-sedation procedures, better device sizing, and fewer repositioning steps, compressing room time and improving lab throughput.
- Safety endpoints under the spotlight. Post-procedure atrial fibrillation, device thrombosis, and nickel sensitivity are managed through device selection, antithrombotic protocols, and follow-up imaging, strengthening payer confidence and guideline concordance.
- Economics beyond the device. Value analysis weighs procedure time, anesthesia, bed utilization, antithrombotic duration, and readmission against outcomes, rewarding platforms that standardize results across operator experience levels.
- Portfolio strategies evolve. Incumbents leverage deep registries and training networks; challengers focus on difficult anatomies, easier recapture, and sheath compatibility to win targeted accounts and expand indications as evidence matures.
- Training and credentialing scale. Proctoring, simulation, and center-of-excellence models reduce variability, enabling safe diffusion from tertiary centers to high-volume community labs with consistent care standards.
- Digital and data integration. Structured reporting, registry participation, and remote follow-up enable continuous learning, earlier detection of complications, and feedback loops for iterative device improvements.
- Materials innovation. Advances in nitinol quality, surface treatments, and bioresorbable layers seek lower thrombogenicity and improved healing, while maintaining recapturability and deliverability through small sheaths.
- Care pathway coordination. Stroke clinics, cardiology, and imaging services integrate to accelerate workup, ensure timely closure, and streamline follow-up, enhancing patient experience and outcome reliability.
PFO Closure Device Market Reginal Analysis
North America
Adoption is characterized by established stroke networks, rigorous value analysis, and broad access to advanced imaging. Hospital systems prioritize platforms with strong safety datasets, straightforward recapture, and compatibility with intracardiac echo for conscious sedation. Private payers and integrated delivery networks emphasize standardized pathways that curb variability and readmissions. Training ecosystems, simulation, and proctoring support diffusion into community cath labs. Post-closure protocols increasingly individualize antithrombotic management based on patient risk, while digital registries capture outcomes and drive continuous quality improvement across multi-site health systems.Europe
Regional practice is shaped by guideline discipline, multidisciplinary boards, and strong emphasis on real-world evidence. Procurement favors devices with proven long-term safety and low residual shunt, validated by independent registries and robust post-market surveillance. Day-case pathways and conscious sedation gain traction in centers with mature imaging capabilities. Reimbursement and tendering processes reward predictable procedure times and comprehensive training support. Environmental and material considerations, including nickel exposure and sustainability initiatives, enter hospital decision criteria alongside clinical metrics.Asia-Pacific
Heterogeneous adoption reflects diverse health-system maturity, with leading centers in developed markets advancing image-guided, sedation-light procedures and structured follow-up. Expanding stroke programs in growth markets drive capacity building, including operator training and protocol standardization. Institutions value sheath compatibility, maneuverability in challenging anatomies, and reliable sealing across variable PFO morphologies. Public-private collaborations and referral networks shorten diagnostic timelines. Portfolio breadth and service responsiveness from suppliers influence wins, particularly where tender processes balance cost with documented outcomes.Middle East & Africa
Selective expansion is linked to investment in cardiac centers, cross-border referrals, and partnerships with global training programs. Hospitals emphasize devices that minimize learning curves and support predictable outcomes with limited imaging resources. Procurement committees weigh durability, follow-up needs, and vendor clinical support. Remote proctoring and visiting-expert models help accelerate competency. Growing stroke awareness and imaging access gradually increase eligibility assessments, while centralized registries begin to track outcomes to inform national policy and reimbursement evolution.South & Central America
Adoption follows the build-out of stroke pathways and structural-heart programs in major urban hospitals. Decision makers prioritize platforms with dependable deliverability, recapture options, and straightforward post-procedure care. Economic assessments consider room turnover, anesthesia models, and follow-up burden, favoring devices that standardize results across operator experience. Supplier partnerships that include training, on-site support, and inventory agility are critical. Regional data collection initiatives and participation in global registries bolster confidence, supporting progressive inclusion in public and private coverage frameworks.PFO Closure Device Market Segmentation
By Type
- Amplatzer PFO Occluder
- Others
By Application
- Hospitals
- Clinics
Key Market players
Abbott, W. L. Gore & Associates, Occlutech, Lifetech Scientific, pfm medical, HeartStitch (NobleStitch EL), Lepu Medical Technology, MicroPort Scientific Corporation, Shanghai Shape Memory Alloy (SHSMA), Starway Medical (Shenzhen Starway Medical Technology), Cardia Inc., Meril Life Sciences, WEGO Group (Shenzhen WEGO Healthcare), Nanjing MDP Medical Technology, Vascular InnovationsPFO Closure Device Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
PFO Closure Device Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - PFO Closure Device market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - PFO Closure Device market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - PFO Closure Device market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - PFO Closure Device market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - PFO Closure Device market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the PFO Closure Device value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the PFO Closure Device industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the PFO Closure Device Market Report
- Global PFO Closure Device market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on PFO Closure Device trade, costs, and supply chains
- PFO Closure Device market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- PFO Closure Device market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term PFO Closure Device market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and PFO Closure Device supply chain analysis
- PFO Closure Device trade analysis, PFO Closure Device market price analysis, and PFO Closure Device supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest PFO Closure Device market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Abbott
- W. L. Gore & Associates
- Occlutech
- Lifetech Scientific
- pfm medical
- HeartStitch (NobleStitch EL)
- Lepu Medical Technology
- MicroPort Scientific Corporation
- Shanghai Shape Memory Alloy (SHSMA)
- Starway Medical (Shenzhen Starway Medical Technology)
- Cardia Inc.
- Meril Life Sciences
- WEGO Group (Shenzhen WEGO Healthcare)
- Nanjing MDP Medical Technology
- Vascular Innovations
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 249.7 Million |
| Forecasted Market Value ( USD | $ 744 Million |
| Compound Annual Growth Rate | 12.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |