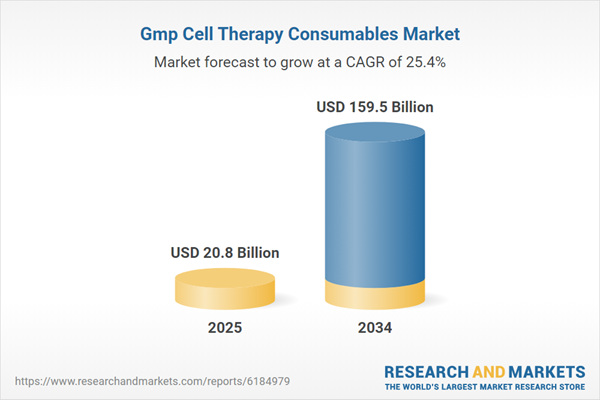
GMP Cell Therapy Consumables Market Overview
The GMP (Good Manufacturing Practice) cell therapy consumables market is witnessing a significant surge, driven by the rising global demand for advanced cell-based treatments and regenerative medicine. These consumables, including media, reagents, sera, and growth factors, are critical to ensuring the safety, consistency, and regulatory compliance of cell therapy manufacturing. With the increasing adoption of personalized medicine and the expanding pipeline of cell-based therapies, manufacturers are scaling up their GMP-compliant production capabilities. Investments from biotech firms and research institutions are further fueling market growth, as companies strive to meet strict regulatory requirements while optimizing cell therapy production processes. The market is particularly expanding across North America and Europe, with Asia-Pacific emerging as a dynamic hub for clinical trials and biomanufacturing. As regulatory bodies continue to tighten standards for cell therapies, demand for high-quality, GMP-grade consumables is anticipated to grow, positioning this market as a vital enabler in the global cell therapy landscape.the GMP cell therapy consumables market experienced a notable shift as major industry players enhanced their focus on automation and closed-system manufacturing to reduce contamination risks. Companies expanded their product lines with xeno-free and serum-free media to align with ethical and regulatory standards, while also catering to a growing range of cell types including CAR-T cells, stem cells, and dendritic cells. Strategic partnerships between CDMOs and biotech innovators accelerated, allowing for more rapid scaling of cell therapy pipelines, particularly for oncology and autoimmune indications. Regulatory authorities such as the FDA and EMA introduced updated guidelines that emphasized the need for traceability, sterility assurance, and batch-to-batch consistency, prompting manufacturers to integrate advanced quality control mechanisms. Additionally, sustainable and single-use technologies gained traction due to their cost-effectiveness and reduced contamination risk, reinforcing a shift toward eco-conscious manufacturing practices in the life sciences industry.
The GMP cell therapy consumables market is expected to benefit from emerging technologies like artificial intelligence (AI)-driven process optimization and digital twins in biomanufacturing. These innovations will enhance consistency and predictive quality control in the production environment. The global market will also witness further regional expansion, particularly in Asia-Pacific and Latin America, as governments invest in life sciences infrastructure and clinical trial support. In parallel, the rise of allogeneic cell therapies and off-the-shelf treatment models will increase the demand for standardized and scalable GMP consumables. Companies are also expected to prioritize end-to-end supply chain solutions, incorporating automation, track-and-trace systems, and robust cold chain logistics. As market competition intensifies, pricing pressures will encourage innovation in cost-effective alternatives without compromising regulatory compliance. Moreover, with the anticipated increase in cell therapy approvals, consumables that support commercialization scale-up and long-term stability testing will become increasingly vital to the market’s growth trajectory.
Key Insights: Gmp Cell Therapy Consumables Market
- Adoption of closed and automated systems in GMP manufacturing is rising to minimize contamination risks and ensure sterility in cell therapy processes.
- Growth in serum-free and chemically defined media products reflects an industry-wide shift toward safer and more ethically sound cell therapy solutions.
- Emerging demand for single-use technologies is transforming biomanufacturing by reducing cleaning validation requirements and enhancing scalability.
- AI-driven quality control and predictive analytics are being explored to improve process consistency and minimize batch failures.
- Collaborations between CDMOs and biotechs are intensifying to accelerate GMP-compliant cell therapy development and commercialization.
- Increasing global investments in cell and gene therapy research are fueling demand for GMP-grade consumables that meet regulatory standards.
- Rising approvals of cell therapy products are encouraging large-scale manufacturing that relies on standardized consumables for reproducibility.
- Growing prevalence of chronic diseases, especially cancer and autoimmune disorders, is driving innovation in cell-based treatment modalities.
- Stricter regulatory frameworks globally are pushing biomanufacturers to adopt high-quality, compliant consumables in their workflows.
- High costs associated with GMP-grade consumables, alongside complex supply chains, pose financial and logistical barriers to smaller biotech firms and startups.
Gmp Cell Therapy Consumables Market Segmentation
By Product
- Kits
- Reagents or Molecular biology reagents
- Growth Factors or Cytokines and Interleukins
- Other Products
By Cell Therapy
- NK Cell Therapy
- Stem Cell Therapy
- T-Cell Therapy
- Other cell therapies
By Process
- Cell Collection and Characterization or Sorting and Separation
- Cell Culture and Expansion or Preparation
- Cryopreservation
- Cell Processing and Formulation
- Cell Isolation and Activation
- Cell Distribution or Handling
- Process Monitoring and Control or Readministration or Quality Assurance
- Other Processes
By End-Use
- Clinical
- Commercial
- Research
Key Companies Analysed
- Thermo Fisher Scientific Inc.
- Fresenius Kabi AG
- Danaher Corporation
- Merck KGaA
- Asahi Kasei Corporation
- GE HealthCare Technologies Inc.
- Corning Incorporated
- Avantor Inc.
- Lonza Group AG
- Terumo Corporation
- Catalent Inc.
- Sartorius AG
- Bio-Techne Corp
- Repligen Corporation
- Miltenyi Biotec BV & Co KG
- Genscript Biotech Corporation
- Rentschler Biopharma SE
- FUJIFILM Irvine Scientific Inc.
- BioLegend Inc.
- STEMCELL Technologies Inc.
- BioLife Solutions Inc.
- Abzena Ltd.
- Sino Biological Inc.
- ProBioGen AG
- MaxCyte Inc.
- PromoCell GmbH
- PeproTech Inc.
- Cellares Corp.
- Wilson Wolf Corporation
- Cellexus Ltd.
Gmp Cell Therapy Consumables Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Gmp Cell Therapy Consumables Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Gmp Cell Therapy Consumables market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Gmp Cell Therapy Consumables market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Gmp Cell Therapy Consumables market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Gmp Cell Therapy Consumables market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Gmp Cell Therapy Consumables market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Gmp Cell Therapy Consumables value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Gmp Cell Therapy Consumables industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Gmp Cell Therapy Consumables Market Report
- Global Gmp Cell Therapy Consumables market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Gmp Cell Therapy Consumables trade, costs, and supply chains
- Gmp Cell Therapy Consumables market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Gmp Cell Therapy Consumables market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Gmp Cell Therapy Consumables market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Gmp Cell Therapy Consumables supply chain analysis
- Gmp Cell Therapy Consumables trade analysis, Gmp Cell Therapy Consumables market price analysis, and Gmp Cell Therapy Consumables supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Gmp Cell Therapy Consumables market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific Inc.
- Fresenius Kabi AG
- Danaher Corporation
- Merck KGaA
- Asahi Kasei Corporation
- GE HealthCare Technologies Inc.
- Corning Incorporated
- Avantor Inc.
- Lonza Group AG
- Terumo Corporation
- Catalent Inc.
- Sartorius AG
- Bio-Techne Corp
- Repligen Corporation
- Miltenyi Biotec BV & Co KG
- Genscript Biotech Corporation
- Rentschler Biopharma SE
- FUJIFILM Irvine Scientific Inc.
- BioLegend Inc.
- STEMCELL Technologies Inc.
- BioLife Solutions Inc.
- Abzena Ltd.
- Sino Biological Inc.
- ProBioGen AG
- MaxCyte Inc.
- PromoCell GmbH
- PeproTech Inc.
- Cellares Corp.
- Wilson Wolf Corporation
- Cellexus Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 20.8 Billion |
| Forecasted Market Value ( USD | $ 159.5 Billion |
| Compound Annual Growth Rate | 25.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |