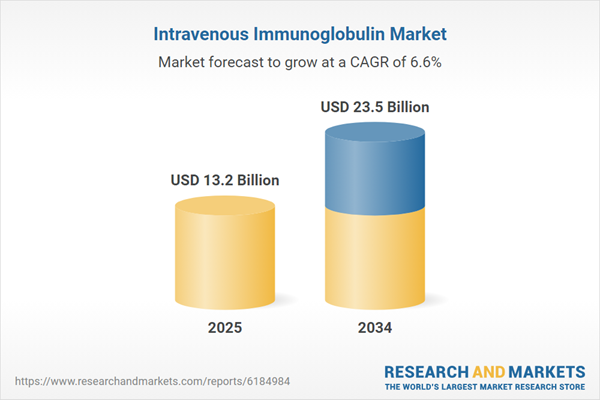
The Intravenous Immunoglobulin (IVIG) Market is dedicated to the production and administration of antibody-rich plasma-derived products used in the treatment of a broad spectrum of immune-related conditions. IVIG therapy is most commonly indicated for primary immunodeficiency disorders, autoimmune diseases such as Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy (CIDP), and certain hematologic conditions like immune thrombocytopenia (ITP). It is also increasingly used off-label for systemic infections, inflammatory responses, and neurologic syndromes. The market has seen substantial growth due to increased awareness of immunoglobulin therapy, improved diagnostic rates for rare diseases, and expansion of IVIG’s therapeutic scope. With plasma collection being the primary source of immunoglobulin, the market remains highly sensitive to donor supply chains and manufacturing capacity. As global demand continues to outpace supply, manufacturers are investing in new plasma fractionation facilities and alternative delivery formulations to meet the rising need.
The IVIG market experienced strong demand growth, particularly in North America and Asia-Pacific. Major producers such as CSL Behring, Grifols, Octapharma, and Takeda increased investments in plasma collection centers to address shortages. Innovations in IVIG delivery - such as higher-concentration formulations and rapid infusion protocols - improved patient compliance and clinic efficiency. Telemedicine platforms began to support remote monitoring for patients receiving long-term IVIG therapy at home, especially those with CIDP and immunodeficiencies. Regulatory agencies updated reimbursement policies in some regions to support IVIG access for newly approved indications. Meanwhile, supply constraints persisted due to complex production timelines and increased reliance on plasma donations. Research into subcutaneous immunoglobulin (SCIG) alternatives continued, though IVIG remained the standard for rapid and high-volume delivery. Hospital purchasing trends showed a shift toward contract agreements and shared networks to secure steady IVIG supply amid global competition.
The IVIG market is poised for further expansion through enhanced plasma processing, alternative delivery methods, and expanded indications. Pharmaceutical companies are expected to focus on optimizing fractionation yield, improving purification technologies, and ensuring traceability across global supply chains. Development of recombinant and synthetic immunoglobulin products may help address long-term supply limitations. IVIG will also see increased use in emerging autoimmune and neurological conditions as clinical evidence broadens. At-home infusion programs supported by digital platforms will scale up, enabling flexible, patient-centered delivery models. Personalized dosing based on weight, biomarkers, and disease severity will become more common as healthcare shifts toward precision medicine. In lower-income countries, international collaborations and policy frameworks will support access to life-saving IVIG therapy for underserved populations. Despite challenges, IVIG’s versatility and efficacy will maintain its role as a cornerstone therapy across immunology and neurology.
Key Insights: Intravenous Immunoglobulin Market
- The analyst highlights the expansion of home-based IVIG infusion programs, driven by the need for patient convenience, reduced hospital exposure, and broader use in chronic autoimmune and neurological conditions.
- High-concentration, rapid-infusion IVIG formulations are trending, offering shorter administration times and improved clinic throughput, especially for high-dose protocols in CIDP and ITP, says the analyst.
- According to the analyst, telehealth integration is increasing in IVIG therapy management, enabling remote monitoring, symptom tracking, and nurse-led support for home-administered infusions.
- The analyst observes growing interest in recombinant or synthetic immunoglobulin research, as the industry seeks to diversify supply sources and reduce reliance on donor plasma over the long term.
- Contract-based purchasing models between hospitals and manufacturers are gaining traction to ensure consistent IVIG supply amid ongoing global plasma shortages and production constraints.
- The analyst identifies increasing prevalence of autoimmune, immunodeficiency, and neurological disorders as the primary demand driver, with IVIG offering proven efficacy across multiple therapeutic areas.
- Rising awareness and improved diagnostics for rare and chronic immune conditions are expanding the patient pool eligible for IVIG therapy, fueling long-term market growth, says the analyst.
- The analyst notes continued investment in plasma collection infrastructure and manufacturing capacity by key players to meet escalating demand and address critical supply shortages.
- The shift toward home-based infusion models and outpatient treatment protocols is accelerating IVIG adoption while reducing strain on hospital resources, according to the analyst.
- The analyst highlights ongoing global plasma supply constraints as a major challenge, with long collection-to-production timelines and donor recruitment issues limiting IVIG availability in many regions.
- According to the analyst, the high cost of IVIG therapy and inconsistent reimbursement across healthcare systems create barriers to access, particularly in lower-income countries and among underinsured patients.
Intravenous Immunoglobulin Market Segmentation
By Type
- IgG (Immunoglobulin G)
- IgM (Immunoglobulin M)
- IgA (Immunoglobulin A)
- IgE (Immunoglobulin E)
- IgD (Immunoglobulin D)
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Other Distribution Channels
By Application
- Hypogammaglobulinemia
- Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
- Primary Immunodeficiency Diseases
- Myasthenia Gravis
- Multifocal Motor Neuropathy
- Other Applications
By End-User
- Hospitals
- Clinics
- Home Care
Key Companies Analysed
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Laboratory Corporation of America Holdings (LabCorp)
- Gentronix Limited (a subsidiary of Eurofins Scientific SE)
- Bio-Rad Laboratories Inc.
- Eurofins Scientific SE
- Charles River Laboratories International Inc.
- Promega Corporation
- Agilent Technologies Inc.
- Abbott Laboratories
- Evotec SE
- SGS SA
- BioIVT LLC
- MB Research Laboratories LLC
- Creative Biolabs Inc.
- Quest Diagnostics Incorporated
- Creative Bioarray Inc.
- InSphero AG
- PerkinElmer Inc.
- Cyprotex PLC
- MatTek Corporation
- Danaher Corporation
- Ncardia AG
- Sekisui Chemical Co. Ltd.
- BioReliance Corporation
- CellSystems Biotechnologie Vertrieb GmbH
- Taconic Biosciences Inc.
- Biopredic International
- MultiCell Technologies Inc.
- Altasciences Clinical Research Holdings Inc.
- Molecular Toxicology Inc.
- Toxys BV
- XenoTech LLC
- Optivia Biotechnology Inc.
- Toxikon Corporation
Intravenous Immunoglobulin Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.
Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.Intravenous Immunoglobulin Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.
Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.Countries Covered
- North America - Intravenous Immunoglobulin market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Intravenous Immunoglobulin market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Intravenous Immunoglobulin market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Intravenous Immunoglobulin market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Intravenous Immunoglobulin market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Intravenous Immunoglobulin value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Intravenous Immunoglobulin industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Intravenous Immunoglobulin Market Report
- Global Intravenous Immunoglobulin market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Intravenous Immunoglobulin trade, costs, and supply chains
- Intravenous Immunoglobulin market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Intravenous Immunoglobulin market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Intravenous Immunoglobulin market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Intravenous Immunoglobulin supply chain analysis
- Intravenous Immunoglobulin trade analysis, Intravenous Immunoglobulin market price analysis, and Intravenous Immunoglobulin supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Intravenous Immunoglobulin market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Laboratory Corporation of America Holdings (LabCorp)
- Gentronix Limited (a subsidiary of Eurofins Scientific SE)
- Bio-Rad Laboratories Inc.
- Eurofins Scientific SE
- Charles River Laboratories International Inc.
- Promega Corporation
- Agilent Technologies Inc.
- Abbott Laboratories
- Evotec SE
- SGS SA
- BioIVT LLC
- MB Research Laboratories LLC
- Creative Biolabs Inc.
- Quest Diagnostics Incorporated
- Creative Bioarray Inc.
- InSphero AG
- PerkinElmer Inc.
- Cyprotex PLC
- MatTek Corporation
- Danaher Corporation
- Ncardia AG
- Sekisui Chemical Co. Ltd.
- BioReliance Corporation
- CellSystems Biotechnologie Vertrieb GmbH
- Taconic Biosciences Inc.
- Biopredic International
- MultiCell Technologies Inc.
- Altasciences Clinical Research Holdings Inc.
- Molecular Toxicology Inc.
- Toxys BV
- XenoTech LLC
- Optivia Biotechnology Inc.
- Toxikon Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 13.2 Billion |
| Forecasted Market Value ( USD | $ 23.5 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 35 |