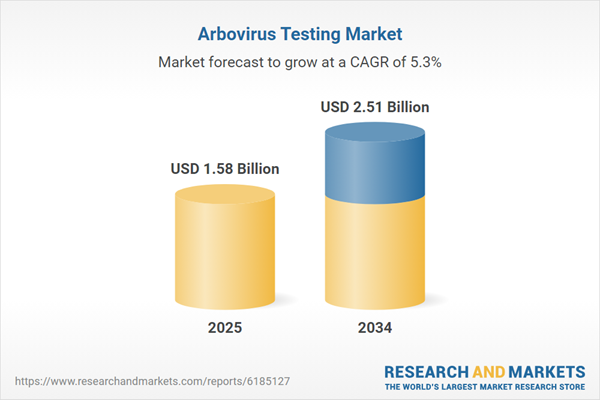
Arbovirus Testing Market
The Arbovirus Testing market covers assays, instruments, consumables, and informatics used to detect mosquito- and tick-borne viruses such as dengue, Zika, chikungunya, West Nile, Japanese encephalitis, and yellow fever. Top applications include acute clinical diagnosis in hospitals and clinics, maternal-fetal screening, blood and organ donor screening, outbreak confirmation and surveillance, traveler health, and vector monitoring. Key trends include rapid decentralization from reference labs to near-patient settings via RT-PCR on portable platforms, isothermal amplification (e.g., LAMP/RPA), high-specificity antigen tests (notably dengue NS1), and multiplex respiratory/fever panels that add arboviruses during transmission seasons. Genomic surveillance - amplicon sequencing, metagenomics, and lineage tracking - now complements diagnostics to inform vector control and vaccine policy. Growth is propelled by climate-driven range expansion of Aedes vectors, urbanization, travel volumes, donor safety requirements, and public-health investment in early warning systems. The competitive landscape blends global IVD majors with regional specialists, competing on analytical sensitivity/specificity across disease day-windows, cross-reactivity control among flaviviruses, turnaround time, and total cost per result under surge demand. Vendors differentiate through room-temperature-stable reagents, closed-tube workflows, digital reporting to surveillance databases, and supply-assurance programs for outbreak spikes. Challenges persist in distinguishing primary vs. secondary flavivirus infections, serology cross-reactivity (including vaccine interference), sample timing constraints (viremia vs. seroconversion), and maintaining quality systems across decentralized sites. Overall, arbovirus testing is shifting from episodic outbreak response to sustained, seasonally tuned, multi-pathogen strategies that integrate molecular, antigen, and serology results with real-time epidemiological data.Arbovirus Testing Market Key Insights
- Seasonal, syndromic strategies win: Health systems increasingly deploy fever/undifferentiated illness panels that toggle arbovirus targets by season and geography, improving case capture without over-testing when prevalence is low.
- Window-aware algorithms matter: Optimal sensitivity requires pairing molecular/antigen tests in early viremia (days 0-7) with IgM/IgG serology thereafter; reflex rules and decision trees reduce false negatives and repeat visits.
- Cross-reactivity is the core challenge: Flavivirus serology (dengue, Zika, yellow fever, JEV) shows antibody cross-binding; confirmatory neutralization tests and orthogonal assays are critical for pregnancy and donor decisions.
- Point-of-care goes molecular: Battery-operable RT-PCR and isothermal platforms with lyophilized reagents extend reliable testing to primary care and field clinics, compressing time-to-result and easing cold-chain burdens.
- Multiplex and workflow integration: Panels that combine dengue/ Zika/ chikungunya (± malaria) on the same run, with auto-interpretation and LIS/LIMS connectivity, streamline triage in fever clinics and emergency departments.
- Genomic surveillance complements diagnostics: Routine sequencing of positives informs lineage dynamics, importation routes, and insecticide resistance correlation - driving targeted vector control and vaccination strategies.
- Donor screening is non-negotiable: Blood and tissue banks adopt seasonal NAT screening or pathogen reduction to mitigate transfusion-transmitted infections, with rapid escalation protocols during outbreaks.
- Quality systems at the edge: Decentralized testing heightens the need for external quality assessment, proficiency panels, and remote instrument monitoring to maintain accuracy during surge staffing.
- Regulation favors readiness: Emergency pathways, pre-positioned validations, and regional reference lab networks enable faster assay activation when incidence rises; suppliers with audit-ready dossiers gain trust.
- Supply resilience is strategic: Dual sourcing of critical oligos/enzymes, reagent kitting at regional hubs, and vendor-managed inventory help laboratories weather demand spikes and logistics disruptions.
Arbovirus Testing Market Reginal Analysis
North America
Demand is driven by seasonal outbreaks (e.g., West Nile, dengue) and robust donor-screening mandates. Public-health labs and hospital networks prioritize multiplex RT-PCR, NS1 antigen tests, and reflex serology algorithms integrated into LIS. Genomic surveillance capacity is strong, informing vector control and travel advisories. Procurement emphasizes FDA-cleared assays, remote instrument management, and surge-ready supply agreements with defined turnaround SLAs.Europe
Most countries rely on centralized reference labs with rapid referral pathways, expanding capacity during imported and autochthonous cases. Travel medicine and blood services maintain targeted NAT and serology workflows. Emphasis on CE-marked assays, cross-reactivity mitigation, and standardized reporting supports cross-border comparability. Vector surveillance and climate-adaptation policies drive investment in integrated testing-genomics platforms.Asia-Pacific
Endemic dengue and periodic chikungunya/JEV activity sustain high baseline testing. Health systems blend rapid antigen tests in primary care with RT-PCR confirmation at district labs; urban centers scale multiplex molecular panels and sequencing. Government programs fund early warning dashboards and school/community screening during peak seasons. Price-performance, reagent stability at high temperatures, and strong distributor support are decisive.Middle East & Africa
Testing demand clusters around urban hubs, humanitarian settings, and cross-border travel corridors. Ministries prioritize simple, robust algorithms: early antigen/RT-PCR with follow-up serology and referral of complex cases to national labs. Donor screening policies vary; regional harmonization is advancing. Procurement favors assays with room-temperature stability, minimal instrumentation, and vendor training to build local competency.South & Central America
High dengue burden drives continuous testing in public and private sectors, with surge protocols during seasonal peaks. Labs deploy NS1/IgM combos at the front line and multiplex RT-PCR at reference centers; sequencing of outbreak strains informs vector campaigns. Budgets and currency volatility shape tenders toward reliable, service-backed platforms. Partnerships with academic consortia strengthen QA programs and data interoperability across states.Arbovirus Testing Market Segmentation
By Product
- Instruments
- Reagents
- Software Services
By Technology
- Immunoassay
- Molecular Diagnostics
- Others
- Microbiology
By Application
- MRSA
- Streptococcus
- Clostridium difficile
- VRE
- CRE
- Respiratory Virus
- Candida
- TB and Drug-resistant TB
- Gastro-intestinal Panel Testing
- Chlamydia
- Gonorrhea
- HPV
- HIV
- Hepatitis C
- Hepatitis B
- COVID-19
- Others
By Location
- Point of Care
- Central Laboratories
- Others
Key Market players
Abbott Laboratories, Thermo Fisher Scientific Inc., F. Hoffmann-La Roche Ltd., QIAGEN N.V., bioMérieux SA, Siemens Healthineers AG, Bio-Rad Laboratories Inc., DiaSorin SpA, NovaTec Immundiagnostica GmbH, Euroimmun AGArbovirus Testing Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Arbovirus Testing Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Arbovirus Testing market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Arbovirus Testing market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Arbovirus Testing market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Arbovirus Testing market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Arbovirus Testing market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Arbovirus Testing value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Arbovirus Testing industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Arbovirus Testing Market Report
- Global Arbovirus Testing market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Arbovirus Testing trade, costs, and supply chains
- Arbovirus Testing market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Arbovirus Testing market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Arbovirus Testing market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Arbovirus Testing supply chain analysis
- Arbovirus Testing trade analysis, Arbovirus Testing market price analysis, and Arbovirus Testing supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Arbovirus Testing market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Thermo Fisher Scientific Inc.
- F. Hoffmann-La Roche Ltd.
- QIAGEN N.V.
- bioMérieux SA
- Siemens Healthineers AG
- Bio-Rad Laboratories Inc.
- DiaSorin SpA
- NovaTec Immundiagnostica GmbH
- Euroimmun AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 1.58 Billion |
| Forecasted Market Value ( USD | $ 2.51 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |