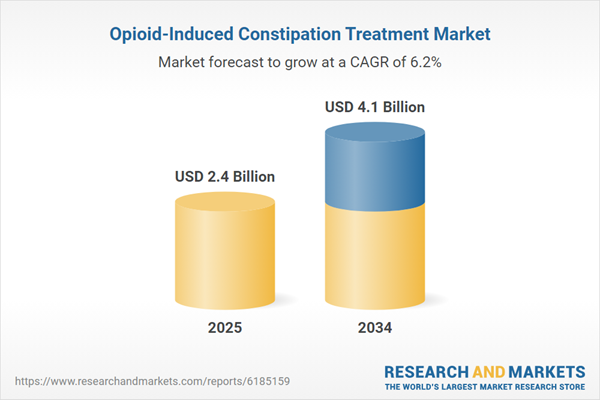
The opioid-induced constipation (OIC) treatment market is expanding due to the rising use of opioid medications for pain management and the growing awareness of opioid-related side effects. OIC is one of the most common and debilitating side effects of long-term opioid use, affecting patients with chronic pain, cancer, and post-surgical conditions. The condition occurs as opioids slow down bowel motility by binding to receptors in the gastrointestinal (GI) tract, making conventional laxatives ineffective in many cases. The market includes prescription drugs such as peripherally acting mu-opioid receptor antagonists (PAMORAs), stool softeners, and lifestyle-based interventions. Pharmaceutical advancements have led to the development of targeted therapies, including naloxegol, methylnaltrexone, and naldemedine, which work by counteracting opioid effects in the gut without compromising pain relief. As opioid prescriptions remain prevalent, demand for effective, well-tolerated OIC treatments continues to rise, prompting drug manufacturers to explore new formulations, alternative mechanisms of action, and combination therapies that enhance bowel function while maintaining patient comfort.
The opioid-induced constipation treatment market witnessed significant growth, driven by increased awareness campaigns, expanded access to prescription treatments, and innovations in drug formulations. New clinical trials demonstrated the efficacy of combination therapies, integrating PAMORAs with traditional laxatives for enhanced relief. The adoption of patient-friendly oral and subcutaneous drug delivery methods increased, offering more convenience for long-term opioid users. Pharmaceutical companies also focused on improving drug tolerability by reducing side effects such as nausea and abdominal pain associated with PAMORAs. Additionally, digital health solutions gained traction, with AI-powered patient monitoring applications helping physicians track OIC symptoms and adjust treatments accordingly. The regulatory landscape saw updates as government agencies prioritized guidelines for managing opioid-related side effects, ensuring broader insurance coverage for OIC-specific treatments. The availability of biosimilars and generic alternatives further increased competition, making these therapies more accessible and cost-effective for a wider range of patients.
The OIC treatment market is expected to advance with the introduction of next-generation therapeutics, personalized medicine approaches, and gut microbiome-targeted therapies. Research into microbiome modulation will play a crucial role in identifying new treatment options that restore gut balance and improve motility without relying solely on receptor antagonists. AI-driven drug development will accelerate the discovery of novel compounds with improved efficacy and fewer side effects. The expansion of precision medicine will enable customized treatment plans based on individual patient genetics, opioid dosage, and gut health, optimizing therapeutic outcomes. Additionally, the pharmaceutical industry will invest in non-opioid pain management alternatives, indirectly impacting OIC prevalence and reshaping the long-term treatment landscape. As healthcare providers continue to prioritize opioid safety and holistic pain management strategies, the demand for effective, minimally invasive, and patient-centric OIC treatments is expected to grow, driving further market expansion.
Key Insights: Opioid-Induced Constipation Treatment Market
Advancements in PAMORA-Based Therapies: The increasing use of peripherally acting mu-opioid receptor antagonists (PAMORAs) is reshaping the OIC treatment landscape by offering targeted relief without compromising pain management. Next-generation PAMORA formulations focus on enhancing bioavailability, minimizing side effects, and providing long-lasting efficacy. Extended-release formulations and novel subcutaneous delivery methods are improving patient adherence, particularly among individuals requiring long-term opioid therapy. As PAMORA-based treatments continue to evolve, they are expected to become the standard of care for patients suffering from opioid-induced constipation.Integration of Digital Health for OIC Management: The adoption of digital health solutions, including AI-powered symptom tracking apps and telemedicine platforms, is revolutionizing OIC treatment. Patients can now log symptoms, medication adherence, and bowel movement patterns, allowing healthcare providers to personalize treatments in real time. AI algorithms analyze patient data to optimize drug dosing, identify potential side effects, and recommend dietary modifications. These digital tools are improving treatment adherence, enhancing physician-patient engagement, and providing valuable insights into the long-term management of opioid-induced constipation.
Increasing Opioid Use in Pain Management: The widespread prescription of opioids for chronic pain, post-surgical recovery, and cancer-related pain management continues to drive demand for effective OIC treatments. Despite efforts to reduce opioid dependency, millions of patients still require these medications for pain relief, necessitating targeted constipation treatments. As healthcare providers focus on opioid safety and patient well-being, OIC therapies are becoming an integral part of comprehensive pain management strategies, ensuring that patients receive adequate relief without compromising gastrointestinal health.
Growing Awareness and Improved Access to OIC Treatments: Increased awareness campaigns by pharmaceutical companies, healthcare organizations, and patient advocacy groups are improving the recognition of OIC as a distinct and treatable condition. Regulatory agencies are also pushing for broader insurance coverage and guideline-based management of opioid-induced constipation. The availability of prescription-based and over-the-counter treatment options, along with telehealth accessibility, is ensuring that more patients receive timely and appropriate interventions, driving growth in the OIC treatment market.
Side Effects and Patient Adherence Issues: Despite the effectiveness of PAMORAs and other OIC treatments, side effects such as nausea, diarrhea, and abdominal discomfort remain a challenge. Many patients hesitate to continue treatment due to these issues, leading to poor adherence and suboptimal outcomes. Addressing this challenge requires continued innovation in drug formulations that maximize efficacy while minimizing adverse effects, ensuring better long-term treatment adherence and patient satisfaction.
Opioid-Induced Constipation Treatment Market Segmentation
By Drug Class
- Laxatives
- Peripherally Acting µ-opioid Receptor Antagonists
- Serotonin Receptor Agonists
- Prostaglandin
By Type
- Oral
- Parenteral
By Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
Key Companies Analysed
- Pfizer Inc.
- Merck & Co. Inc.
- Bayer AG
- Sanofi S.A.
- AstraZeneca plc
- Takeda Pharmaceutical Company Limited
- Daiichi Sankyo Chemical Pharma Co. Ltd.
- Bausch Health Companies Inc.
- Boehringer Ingelheim International GmbH.
- Shionogi & Co. Ltd.
- Ono Pharmaceutical Co. Ltd.
- Dr. Reddy's Laboratories Ltd.
- Hikma Pharmaceuticals plc
- Mundipharma International Limited
- Lantheus Holdings Inc.
- Indivior plc
- Mallinckrodt Pharmaceuticals
- Collegium Pharmaceutical Inc.
- Ironwood Pharmaceuticals Inc.
- Cosmo Pharmaceuticals SA
- Nektar Therapeutics
- RedHill Biopharma Ltd.
- Theravance Biopharma Inc.
- Cumberland Pharmaceuticals Inc.
- Valinor Pharma LLC
Opioid-Induced Constipation Treatment Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Opioid-Induced Constipation Treatment Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Opioid-Induced Constipation Treatment market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Opioid-Induced Constipation Treatment market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Opioid-Induced Constipation Treatment market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Opioid-Induced Constipation Treatment market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Opioid-Induced Constipation Treatment market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Opioid-Induced Constipation Treatment value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Opioid-Induced Constipation Treatment industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Opioid-Induced Constipation Treatment Market Report
- Global Opioid-Induced Constipation Treatment market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Opioid-Induced Constipation Treatment trade, costs, and supply chains
- Opioid-Induced Constipation Treatment market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Opioid-Induced Constipation Treatment market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Opioid-Induced Constipation Treatment market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Opioid-Induced Constipation Treatment supply chain analysis
- Opioid-Induced Constipation Treatment trade analysis, Opioid-Induced Constipation Treatment market price analysis, and Opioid-Induced Constipation Treatment supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Opioid-Induced Constipation Treatment market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Merck & Co. Inc.
- Bayer AG
- Sanofi S.A.
- AstraZeneca PLC
- Takeda Pharmaceutical Company Limited
- Daiichi Sankyo Chemical Pharma Co. Ltd.
- Bausch Health Companies Inc.
- Boehringer Ingelheim International GmbH.
- Shionogi & Co. Ltd.
- Ono Pharmaceutical Co. Ltd.
- Dr. Reddy's Laboratories Ltd.
- Hikma Pharmaceuticals PLC
- Mundipharma International Limited
- Lantheus Holdings Inc.
- Indivior PLC
- Mallinckrodt Pharmaceuticals
- Collegium Pharmaceutical Inc.
- Ironwood Pharmaceuticals Inc.
- Cosmo Pharmaceuticals SA
- Nektar Therapeutics
- RedHill Biopharma Ltd.
- Theravance Biopharma Inc.
- Cumberland Pharmaceuticals Inc.
- Valinor Pharma LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 2.4 Billion |
| Forecasted Market Value ( USD | $ 4.1 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |