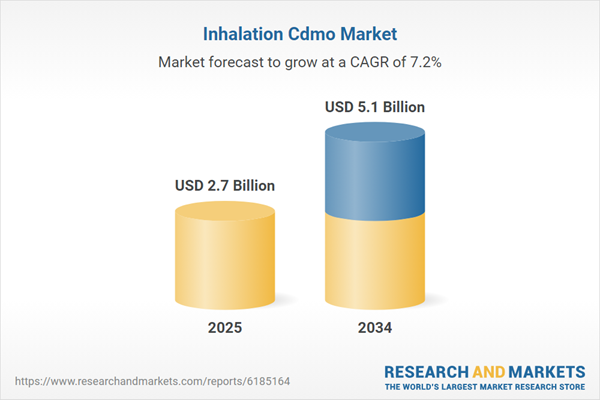
The inhalation contract development and manufacturing organization (CDMO) market is experiencing substantial growth, driven by the increasing prevalence of respiratory diseases like asthma and chronic obstructive pulmonary disease (COPD). This market caters to pharmaceutical and biotech companies seeking specialized expertise in developing and manufacturing inhalation drug products, including metered-dose inhalers (MDIs), dry powder inhalers (DPIs), and nebulizers. CDMOs offer comprehensive services, from formulation development and analytical testing to clinical trial manufacturing and commercial-scale production. The complexity of inhalation drug delivery systems, coupled with stringent regulatory requirements, necessitates specialized knowledge and facilities, making CDMOs crucial partners for pharmaceutical companies. The market's expansion is further propelled by the rising demand for biologics and biosimilars in respiratory therapies, as well as the growing trend towards outsourcing manufacturing activities to reduce costs and improve efficiency. The focus on patient-centric drug delivery and the development of novel inhalation technologies are also shaping the market landscape. The need for consistent and high quality inhalation products is also a large factor in the markets growth.
The inhalation CDMO market witnessed significant advancements in formulation development and manufacturing technologies. The integration of advanced analytical techniques, such as particle size analysis and aerodynamic particle size distribution (APSD) testing, has improved the characterization and quality control of inhalation products. The market also saw an increased focus on developing sustainable and environmentally friendly manufacturing processes, driven by growing regulatory pressures and consumer awareness. Furthermore, the adoption of digital technologies, such as process analytical technology (PAT) and real-time monitoring systems, has enhanced process efficiency and product consistency. The demand for CDMO services for biologics and biosimilars in respiratory therapies continued to rise, reflecting the growing pipeline of these complex drug products. Additionally, the market experienced a surge in demand for CDMOs with expertise in developing and manufacturing novel inhalation devices, such as smart inhalers and connected health solutions. The impact of regulatory changes and the increasing need for supply chain resilience also influenced market dynamics in 2024. The CDMOs that were able to handle the complexity and regulatory burden saw larger market share gains.
The inhalation CDMO market is expected to maintain its growth trajectory, driven by continued innovation and evolving patient needs. The development of personalized inhalation therapies, tailored to individual patient profiles, is anticipated to gain momentum, requiring CDMOs to offer flexible and scalable manufacturing solutions. The integration of artificial intelligence (AI) and machine learning (ML) in formulation development and manufacturing processes is expected to enhance efficiency and reduce development timelines. Furthermore, the market is likely to witness an increased focus on developing and manufacturing inhaled gene therapies and cell therapies for respiratory diseases. The adoption of advanced manufacturing technologies, such as continuous manufacturing and 3D printing, is expected to improve production efficiency and reduce costs. The expansion of CDMO services for emerging markets, where respiratory diseases are prevalent, will also contribute to market growth. The evolving regulatory landscape and the increasing demand for high-quality, patient-centric inhalation products will continue to shape the market's future. The increasing use of connected devices and digital therapeutics will drive more complexity into the CDMO offerings.
Key Insights: Inhalation Cdmo Market
- Increased Demand for Biologics and Biosimilars: The growing pipeline of biologics and biosimilars for respiratory therapies is driving the demand for CDMOs with specialized expertise in handling these complex molecules.
- Focus on Patient-Centric Drug Delivery: The development of novel inhalation devices, such as smart inhalers and connected health solutions, is enhancing patient adherence and improving therapeutic outcomes.
- Adoption of Advanced Analytical Techniques: The use of particle size analysis, APSD testing, and other advanced analytical methods is improving the characterization and quality control of inhalation products.
- Emphasis on Sustainable Manufacturing: Growing regulatory pressures and consumer awareness are driving the adoption of environmentally friendly manufacturing processes and technologies.
- Integration of Digital Technologies: The implementation of PAT, real-time monitoring systems, and other digital tools is enhancing process efficiency and product consistency.
- Rising Prevalence of Respiratory Diseases: The increasing incidence of asthma, COPD, and other respiratory conditions is driving the demand for effective inhalation therapies.
- Growing Demand for Outsourcing Manufacturing: Pharmaceutical companies are increasingly outsourcing manufacturing activities to CDMOs to reduce costs, improve efficiency, 1 and access specialized expertise.
- Technological Advancements in Inhalation Devices: The development of novel inhalation technologies, such as smart inhalers and nebulizers, is expanding the market for CDMO services.
- Stringent Regulatory Requirements: The complex regulatory landscape for inhalation drug products necessitates specialized expertise, driving the demand for CDMO services.
- Complexity of Formulation Development: Inhalation drug products require precise formulation and particle engineering, posing significant challenges for CDMOs.
- High Manufacturing Costs: The specialized equipment and facilities required for manufacturing inhalation products can lead to high production costs, limiting market accessibility.
Inhalation Cdmo Market Segmentation
By Product
- Dry Powder Inhaler (DPIs)
- Metered Dose Inhaler (MDIs)
- Nebulizer
- Soft Mist Inhaler
By Disease Indication
- Asthma
- Chronic Obstructive Pulmonary Disease (COPD)
- Pulmonary Arterial Hypertension
- Acute Respiratory Distress Syndrome
- Pulmonary Fibrosis
By Technology
- Manual Inhalers (SMI)
- Digital Inhalers
By End-User
- Big Pharmaceutical Companies
- Small and Medium-sized Pharmaceutical Companies
- Generic Pharmaceutical Companies
- Other End-Users
Key Companies Analysed
- Pfizer Inc.
- Merck KGaA
- Baxter’s BioPharma Solutions
- Lonza Group AG
- Catalent Inc.
- AptarGroup Inc.
- Samsung Biologics Co. Ltd.
- Siegfried Holding AG
- Recipharm AB
- Stevanato Group SpA
- Cambrex Corporation
- CordenPharma
- Nemera
- Hovione FarmaCiencia SA
- Vectura Group Ltd.
- CARBOGEN AMCIS
- Kindeva Drug Delivery Ltd.
- Frontage Laboratories Inc
- Formosa Laboratories Inc
- Sanner GmbH
- H&T Presspart Ltd.
- Astech Projects Ltd
- Cliantha Research
- Dipharma Francis Srl
- Copley Scientific
Inhalation Cdmo Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Inhalation Cdmo Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Inhalation Cdmo market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Inhalation Cdmo market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Inhalation Cdmo market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Inhalation Cdmo market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Inhalation Cdmo market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Inhalation Cdmo value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Inhalation Cdmo industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Inhalation Cdmo Market Report
- Global Inhalation Cdmo market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Inhalation Cdmo trade, costs, and supply chains
- Inhalation Cdmo market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Inhalation Cdmo market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Inhalation Cdmo market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Inhalation Cdmo supply chain analysis
- Inhalation Cdmo trade analysis, Inhalation Cdmo market price analysis, and Inhalation Cdmo supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Inhalation Cdmo market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Merck KGaA
- Baxter’s BioPharma Solutions
- Lonza Group AG
- Catalent Inc.
- AptarGroup Inc.
- Samsung Biologics Co. Ltd.
- Siegfried Holding AG
- Recipharm AB
- Stevanato Group SpA
- Cambrex Corporation
- CordenPharma
- Nemera
- Hovione FarmaCiencia SA
- Vectura Group Ltd.
- CARBOGEN AMCIS
- Kindeva Drug Delivery Ltd.
- Frontage Laboratories Inc.
- Formosa Laboratories Inc.
- Sanner GmbH
- H&T Presspart Ltd.
- Astech Projects Ltd.
- Cliantha Research
- Dipharma Francis Srl
- Copley Scientific
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 2.7 Billion |
| Forecasted Market Value ( USD | $ 5.1 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |