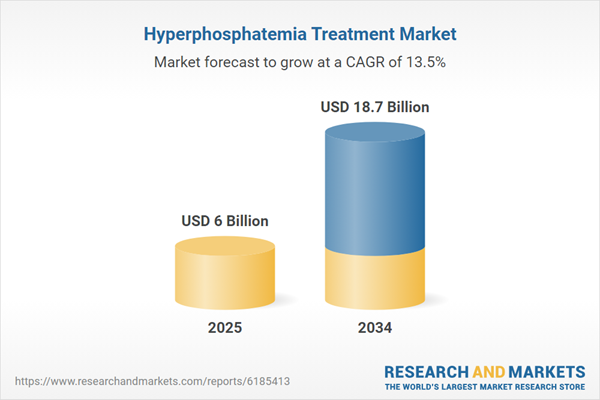
The Hyperphosphatemia Treatment Market addresses the clinical management of elevated phosphate levels in patients, primarily those with chronic kidney disease (CKD). Hyperphosphatemia is a serious complication in end-stage renal disease, often leading to cardiovascular disorders and bone-related complications. The market comprises phosphate binders, dietary management strategies, and ongoing innovations in dialysis techniques. Major pharmaceutical companies such as Sanofi, Fresenius, and Vifor Pharma are active players, developing advanced formulations that reduce pill burden and improve gastrointestinal tolerability. With the global increase in CKD prevalence, particularly due to aging populations and lifestyle-related conditions like diabetes and hypertension, the need for effective, patient-centric phosphate management is growing. The market is highly reliant on prescription therapeutics, and ongoing clinical research is aimed at improving safety, efficacy, and compliance.
The hyperphosphatemia treatment market saw notable developments in both drug formulation and patient care strategies. Several manufacturers introduced chewable or gel-based binders to enhance palatability and reduce treatment fatigue. Combination therapies with iron-based binders gained favor, particularly for anemic CKD patients. Guidelines issued by nephrology associations emphasized early intervention, leading to broader adoption of phosphate control in stage 3-4 CKD rather than just dialysis-dependent cases. Market expansion was evident across Asia-Pacific and Latin America, where awareness campaigns and access to CKD screening improved diagnosis rates. Additionally, digital tools for diet tracking and treatment reminders began to complement traditional therapies, supporting better adherence and overall phosphate control outcomes.
The market is expected to benefit from increased investment in biologics and gene therapies targeting mineral metabolism pathways. Oral binders will continue to evolve with fewer side effects, improved bioavailability, and once-daily dosing formulations. Additionally, AI-powered health monitoring tools may enable personalized phosphate control plans by integrating lab data, dietary intake, and patient behavior patterns. The trend toward value-based healthcare will pressure pharmaceutical firms to demonstrate real-world outcomes, prompting more longitudinal studies and partnerships with nephrology networks. As CKD prevalence continues to rise, especially in aging populations and developing nations, demand for both traditional and novel hyperphosphatemia treatments will remain strong. Regulatory bodies are expected to streamline approval pathways for innovative therapies to address the unmet clinical need more efficiently.
Key Insights: Hyperphosphatemia Treatment Market
- Development of chewable, liquid, and gel-based phosphate binders is improving compliance among patients with swallowing difficulties and pill fatigue.
- Integration of digital tools for diet tracking and treatment reminders is supporting better patient adherence and phosphate level monitoring.
- Iron-based combination binders are gaining traction due to dual benefits in managing phosphate and addressing anemia in CKD patients.
- Shift toward early-stage CKD management is expanding the treatment-eligible population beyond dialysis-dependent individuals.
- Clinical research is exploring novel biologics and gene therapies to address phosphate regulation at the metabolic level.
- Rising global prevalence of chronic kidney disease and associated complications is expanding the target population for phosphate management therapies.
- Ongoing innovation in drug formulations is reducing pill burden and improving patient quality of life and treatment adherence.
- Greater clinical awareness and revised treatment guidelines are driving earlier diagnosis and proactive management of hyperphosphatemia.
- Healthcare digitization is enabling personalized treatment plans and remote patient engagement in chronic disease management.
- High treatment costs and limited accessibility in low-resource settings restrict widespread adoption of advanced phosphate binders and CKD therapies.
Hyperphosphatemia Treatment Market Segmentation
By Product
- Sevelamer
- Calcium-Based Phosphate Binders
- Iron-Based Phosphate Binders
- Lanthanum Carbonate
- Other products
By Distribution channel
- Hospital Pharmacy
- Retail Pharmacy
- Online stores
Key Companies Analysed
- Sanofi SA
- Akebia Therapeutics Inc.
- AMAG Pharmaceuticals Inc.
- Ardelyx Inc.
- Astellas Pharma Inc.
- Fresenius Medical Care AG & Co. KGaA
- Keryx Biopharmaceuticals Inc.
- Lupin Limited
- Shire plc
- Sun Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company Limited
- Ultragenyx Pharmaceutical Inc.
- Unicycive Therapeutics Inc.
- Vifor Pharma Management Ltd.
- Zeria Pharmaceutical Co. Ltd.
- Abbott Laboratories
- Amgen Inc.
- AstraZeneca plc
- B. Braun Melsungen AG
- Baxter International Inc.
- Beckman Coulter Inc.
- Cleveland Clinic
- DaVita Inc.
- Encompass Health Corporation
- FMC Corporation
- Hospital Corporation of America Healthcare Inc.
- Kyowa Kirin Pharmaceuticals Co. Ltd.
- Mayo Clinic
- Medtronic plc
- OPKO Health Inc.
- Roche Diagnostics Corporation
- Siemens Healthineers AG
- Sysmex Corporation
- Tenet Healthcare Corporation
- Thermo Fisher Scientific Inc.
- UnitedHealth Group Inc.
Hyperphosphatemia Treatment Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Hyperphosphatemia Treatment Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Hyperphosphatemia Treatment market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Hyperphosphatemia Treatment market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Hyperphosphatemia Treatment market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Hyperphosphatemia Treatment market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Hyperphosphatemia Treatment market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Hyperphosphatemia Treatment value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Hyperphosphatemia Treatment industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Hyperphosphatemia Treatment Market Report
- Global Hyperphosphatemia Treatment market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Hyperphosphatemia Treatment trade, costs, and supply chains
- Hyperphosphatemia Treatment market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Hyperphosphatemia Treatment market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Hyperphosphatemia Treatment market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Hyperphosphatemia Treatment supply chain analysis
- Hyperphosphatemia Treatment trade analysis, Hyperphosphatemia Treatment market price analysis, and Hyperphosphatemia Treatment supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Hyperphosphatemia Treatment market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Sanofi SA
- Akebia Therapeutics Inc.
- AMAG Pharmaceuticals Inc.
- Ardelyx Inc.
- Astellas Pharma Inc.
- Fresenius Medical Care AG & Co. KGaA
- Keryx Biopharmaceuticals Inc.
- Lupin Limited
- Shire PLC
- Sun Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company Limited
- Ultragenyx Pharmaceutical Inc.
- Unicycive Therapeutics Inc.
- Vifor Pharma Management Ltd.
- Zeria Pharmaceutical Co. Ltd.
- Abbott Laboratories
- Amgen Inc.
- AstraZeneca PLC
- B. Braun Melsungen AG
- Baxter International Inc.
- Beckman Coulter Inc.
- Cleveland Clinic
- DaVita Inc.
- Encompass Health Corporation
- FMC Corporation
- Hospital Corporation of America Healthcare Inc.
- Kyowa Kirin Pharmaceuticals Co. Ltd.
- Mayo Clinic
- Medtronic PLC
- OPKO Health Inc.
- Roche Diagnostics Corporation
- Siemens Healthineers AG
- Sysmex Corporation
- Tenet Healthcare Corporation
- Thermo Fisher Scientific Inc.
- UnitedHealth Group Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 6 Billion |
| Forecasted Market Value ( USD | $ 18.7 Billion |
| Compound Annual Growth Rate | 13.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 36 |