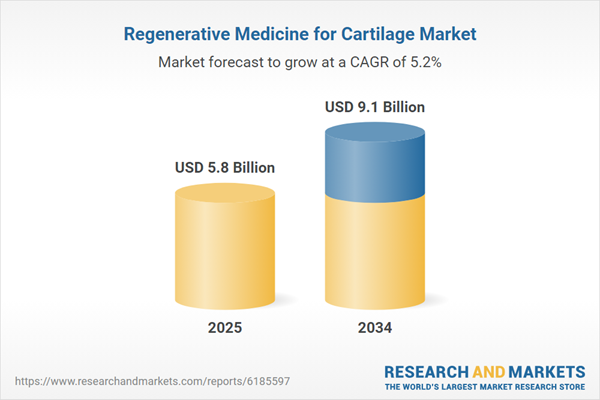
The Regenerative Medicine for Cartilage Market is emerging as a transformative solution for treating cartilage damage and degenerative joint conditions such as osteoarthritis, trauma-induced injuries, and sports-related cartilage wear. Cartilage, having limited self-repair capacity, often leads to chronic pain and reduced mobility when damaged. Regenerative medicine offers hope through advanced therapies that restore or replace damaged cartilage using stem cells, tissue engineering, gene therapy, and biomaterials. This market includes autologous chondrocyte implantation (ACI), mesenchymal stem cell (MSC)-based therapies, and 3D bioprinted cartilage scaffolds. Growing elderly populations, rising incidence of joint disorders, and increasing sports injuries are key drivers of demand. Additionally, patients’ preference for minimally invasive and biologically compatible alternatives to joint replacements has accelerated clinical research and commercialization efforts. As technology advances and clinical outcomes improve, the regenerative cartilage market is transitioning from experimental applications to scalable clinical treatments with significant long-term potential.
The regenerative medicine for cartilage market experienced notable advancements in clinical development, commercialization, and regulatory progress. Several late-stage clinical trials for stem cell-based and tissue-engineered cartilage repair therapies yielded promising results, attracting investor confidence and fast-track designations from regulatory bodies in the U.S. and Europe. Biotech companies and academic institutions collaborated to refine scaffold designs and optimize cell delivery methods, improving the structural integrity and longevity of regenerated cartilage. Orthopedic centers increasingly adopted biologic therapies for early-stage joint degeneration, particularly in younger, active patients. In Asia-Pacific, government-supported innovation programs facilitated rapid growth in regenerative therapy infrastructure, making advanced treatments more accessible. Meanwhile, digital tools for joint health monitoring and outcome tracking became part of post-treatment protocols, enhancing the integration of regenerative medicine into mainstream orthopedic care. The year marked a critical shift toward validated, patient-ready solutions and growing physician confidence in the safety and efficacy of cartilage regeneration therapies.
The regenerative medicine for cartilage market is expected to mature further as new therapies gain regulatory approval and enter routine clinical practice. Emerging technologies such as 3D bioprinting and gene editing will enhance personalization and repair efficacy, enabling custom cartilage implants tailored to individual joint geometry and tissue dynamics. The introduction of allogeneic off-the-shelf stem cell therapies will reduce treatment costs and improve accessibility, particularly in outpatient settings. Collaborations between pharma companies, device manufacturers, and healthcare providers will lead to integrated treatment platforms combining diagnostics, biologics, and rehabilitation support. Regulatory frameworks are likely to evolve, standardizing safety and manufacturing guidelines for regenerative cartilage products. In parallel, patient awareness and demand will increase as more long-term data confirms durability and improved mobility compared to conventional surgical options. As regenerative medicine reshapes orthopedic care, cartilage therapies will become a cornerstone for treating joint degeneration and preserving mobility in aging populations.
Key Insights: Regenerative Medicine For Cartilage Market
- Autologous and allogeneic stem cell therapies for cartilage regeneration are gaining clinical momentum, offering minimally invasive alternatives to joint replacement surgeries for early-stage joint damage.
- 3D bioprinting is being explored to develop patient-specific cartilage constructs, improving tissue compatibility and repair outcomes in orthopedic applications.
- Tissue-engineered scaffolds made from biomaterials like collagen, hyaluronic acid, and hydrogels are being refined for enhanced cell attachment, growth, and long-term integration.
- Digital health platforms and joint monitoring apps are being integrated into regenerative therapy programs to track patient outcomes and personalize rehabilitation plans.
- Global collaborations among biotech firms, orthopedic specialists, and academic institutions are accelerating R&D, clinical trials, and regulatory pathways for novel cartilage therapies.
- Rising prevalence of osteoarthritis and sports-related cartilage injuries is increasing demand for non-invasive, regenerative treatment alternatives that can delay or avoid joint replacement surgeries.
- Growing aging population worldwide is fueling the need for mobility-preserving interventions that support joint health and reduce long-term disability.
- Advancements in stem cell biology, tissue engineering, and biomaterials are improving the safety, efficacy, and scalability of regenerative cartilage repair solutions.
- Supportive regulatory policies, fast-track designations, and reimbursement expansions are encouraging the commercialization of regenerative therapies for orthopedic conditions.
- High treatment costs, lack of standardized protocols, and limited insurance coverage remain significant barriers to widespread adoption of regenerative cartilage therapies, especially in public healthcare systems and price-sensitive markets.
Regenerative Medicine For Cartilage Market Segmentation
By Treatment Modality
- Cell-Based
- Non-Cell-Based
By Treatment Type
- Palliative
- Intrinsic Repair Stimulus
- Others Skin Resurfacing
By Site
- Knee Cartilage Repair
- Ribs
By Application
- Hyaline Cartilage Repair and Regeneration
- Elastic Cartilage Repair and Regeneration
- Fibrous Cartilage Repair and Regeneration
By End-User: Ambulatory Surgical Centers
- Hospitals & Clinics
Key Companies Analysed
- Smith & Nephew plc
- Integra LifeSciences
- Stryker Corporation
- MiMedx Group
- Inc.
- Zimmer Biomet Holdings Inc.
- B. Braun Melsungen AG
- AbbVie
- CONMED Corporation
- Arthrex Inc.
- Baxter International Inc.
- China Regenerative Medicine International
- AstraZenaca
- ABLbio
- Adimmune
- Henlius
- Innovent Biologics
- Bio-Thera
- Hisun Pharma
- 3SBio
- Biocon
- Dr.Reddy’s
- Mylan
- Kyowa Hakko Kirin
- Takeda
- Mitsubishi Tanabe
- Cellgenix Gmbh
- BioTissue Technologies
- Corin Group
- Medical Import Ltd.
- Lucideon
- Orthonika
- Merete
- 3di GmbH
- Aditus Medical GmbH
- Anton Hipp GmbH
- AREX SAS
- Acelity L.P. Inc.
- Celgene Corporation
- StemCells
- Inc
- Organogenesis Inc
- NuVasive
- Inc
- Japan Tissue Engineering Co.
- Ltd
- Advantagene
- Inc.
- Mesoblast Ltd
- Globus Medical
- DePuy Synthes
- Cryolife Inc
- Johnson & Johnson
- Medtronic Plc
- Rti Surgical
- CartiHeal
- Inc.
- DJO Global Inc.
- Wright Medical Group
- Auxein
- Aesculap
- Inc
- AMEGA Biotech
- BioMarin Pharmaceutical
- Ache Laboratorios Farmaceuticos
- Myralis Industria Farmaceuticaa
- Biosonda Biotechnology
- Ipsum Clinical
- Steris Applied Sterilization Technologies
Regenerative Medicine For Cartilage Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Regenerative Medicine For Cartilage Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Regenerative Medicine For Cartilage market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Regenerative Medicine For Cartilage market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Regenerative Medicine For Cartilage market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Regenerative Medicine For Cartilage market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Regenerative Medicine For Cartilage market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Regenerative Medicine For Cartilage value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Regenerative Medicine For Cartilage industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Regenerative Medicine For Cartilage Market Report
- Global Regenerative Medicine For Cartilage market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Regenerative Medicine For Cartilage trade, costs, and supply chains
- Regenerative Medicine For Cartilage market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Regenerative Medicine For Cartilage market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Regenerative Medicine For Cartilage market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Regenerative Medicine For Cartilage supply chain analysis
- Regenerative Medicine For Cartilage trade analysis, Regenerative Medicine For Cartilage market price analysis, and Regenerative Medicine For Cartilage supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Regenerative Medicine For Cartilage market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Smith & Nephew PLC
- Integra LifeSciences
- Stryker Corporation
- MiMedx Group Inc.
- Zimmer Biomet Holdings Inc.
- B. Braun Melsungen AG
- AbbVie
- CONMED Corporation
- Arthrex Inc.
- Baxter International Inc.
- China Regenerative Medicine International
- AstraZenaca
- ABLbio
- Adimmune
- Henlius
- Innovent Biologics
- Bio-Thera
- Hisun Pharma
- 3SBio
- Biocon
- Dr.Reddy’s
- Mylan
- Kyowa Hakko Kirin
- Takeda
- Mitsubishi Tanabe
- Cellgenix Gmbh
- BioTissue Technologies
- Corin Group
- Medical Import Ltd.
- Lucideon
- Orthonika
- Merete
- 3di GmbH
- Aditus Medical GmbH
- Anton Hipp GmbH
- AREX SAS
- Acelity L.P. Inc.
- Celgene Corporation
- StemCells Inc.
- Organogenesis Inc.
- NuVasive Inc.
- Japan Tissue Engineering Co. Ltd.
- Advantagene Inc.
- Mesoblast Ltd.
- Globus Medical
- DePuy Synthes
- Cryolife Inc.
- Johnson & Johnson
- Medtronic PLC
- Rti Surgical
- CartiHeal Inc.
- DJO Global Inc.
- Wright Medical Group
- Auxein
- Aesculap Inc.
- AMEGA Biotech
- BioMarin Pharmaceutical
- Ache Laboratorios Farmaceuticos
- Myralis Industria Farmaceuticaa
- Biosonda Biotechnology
- Ipsum Clinical
- Steris Applied Sterilization Technologies
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 5.8 Billion |
| Forecasted Market Value ( USD | $ 9.1 Billion |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 62 |