The Implantable Cardiac Rhythm Management (CRM) Device Market is a vital segment in the cardiovascular medical device industry, focused on the development and use of devices that monitor and regulate the heart's rhythm. These devices include pacemakers, defibrillators, and implantable cardioverter defibrillators (ICDs), all designed to treat arrhythmias and other heart rhythm disorders. CRM devices are critical for patients suffering from conditions such as bradycardia, tachycardia, or sudden cardiac arrest. As the global prevalence of heart disease rises and healthcare advances, the demand for implantable cardiac devices continues to grow. The market is also driven by technological innovations that have improved the longevity, safety, and functionality of CRM devices, making them more accessible and effective for patients.
The implantable cardiac rhythm management device market saw significant developments with the introduction of advanced features such as remote monitoring capabilities and improved battery life. The shift towards more patient-centric care models, where CRM devices can send real-time data to healthcare providers for continuous monitoring, became more prevalent. This has enabled early intervention in case of abnormalities and improved patient outcomes. Moreover, innovations in leadless pacemakers and miniaturization of devices allowed for less invasive procedures and faster recovery times. The use of artificial intelligence (AI) and machine learning (ML) in device programming and predictive analytics also gained traction, improving device personalization and treatment effectiveness. The regulatory environment continued to evolve, ensuring that CRM devices meet safety and efficacy standards, which in turn encouraged further market growth.
The implantable cardiac rhythm management device market is expected to witness continued advancements in device capabilities, particularly with the integration of next-generation technologies such as wireless communication, bioelectronics, and AI-driven algorithms. These innovations will allow CRM devices to become more intelligent, capable of automatically adjusting to a patient's needs and providing deeper insights into heart health. Moreover, the rise of miniaturized devices, coupled with improvements in energy efficiency and battery life, will expand the use of CRM devices to a broader patient population. The increasing trend toward outpatient procedures and home monitoring will drive the development of more accessible and affordable CRM devices, allowing for greater patient autonomy in managing their heart health.
Key Insights: Implantable Cardiac Rhythm Management Device Market
- Advancements in remote monitoring and wireless communication capabilities, enabling continuous heart health tracking and early intervention.
- Miniaturization and less invasive procedures, such as leadless pacemakers, making implantable CRM devices more accessible and reducing recovery times.
- Integration of artificial intelligence (AI) and machine learning (ML) in device programming, enabling more personalized and adaptive treatment plans for patients.
- Growing demand for long-lasting, energy-efficient devices with improved battery life to increase the duration between device replacements.
- Increasing shift toward outpatient procedures and home monitoring, empowering patients to manage their heart health with more autonomy.
- The rising global prevalence of heart disease and arrhythmias is increasing the demand for implantable cardiac rhythm management devices.
- Technological advancements in device design, including improved performance, smaller size, and better battery life, are driving market growth.
- Growing patient preference for minimally invasive procedures and quicker recovery times is leading to higher adoption of implantable CRM devices.
- The increasing focus on personalized medicine and predictive analytics is enhancing the effectiveness and customization of CRM treatments.
- High costs associated with implantable cardiac devices and long-term maintenance can limit accessibility, particularly in emerging markets.
- Complications related to device implantation, such as infections and lead displacement, remain concerns in the broader adoption of CRM devices.
Implantable Cardiac Rhythm Management Device Market Segmentation
By Device
- Cardiac Resynchronization Therapy
- Defibrillators
- Pacemakers
By Application
- Bradycardia
- Tachycardia
- Other Applications
By End-Use
- Hospitals
- Specialty Cardiac Centers
- Other End-Uses
Key Companies Analysed
- Stryker Corporation
- Schiller AG
- Medtronic plc
- Abbott Laboratories
- Boston Scientific Corporation
- Koninklijke Philips N.V.
- Zoll Medical Corporation
- Biotronik Ltd.
- MicroPort Scientific Corporation
- Abiomed Inc
- Hydrix Limited
- Nihon Kohden Corporation
- Japan Lifeline Co. Ltd.
- Lepu Medical Technology Co. Ltd.
- LivaNova PLC
- Shenzhen Mindray Biomedical Electronics Co. Ltd
- Progetti Srl
- Edwards Lifesciences Corporation
- Cardiac Science Corporation
- Sorin Group S.p.A.
- St. Jude Medical LLC
- Osprey Medical Inc.
- Bardy Diagnostics Inc.
- VivaLNK Inc.
- NanoMatriX International Limited
- Beijing Demax Medical Technology Co. Ltd
- iRhythm Technologies Inc.
- Impulse Dynamics plc.
- SentiAR Inc.
- VytronUS Inc.
Implantable Cardiac Rhythm Management Device Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Implantable Cardiac Rhythm Management Device Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Implantable Cardiac Rhythm Management Device market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Implantable Cardiac Rhythm Management Device market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Implantable Cardiac Rhythm Management Device market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Implantable Cardiac Rhythm Management Device market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Implantable Cardiac Rhythm Management Device market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Implantable Cardiac Rhythm Management Device value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Implantable Cardiac Rhythm Management Device industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Implantable Cardiac Rhythm Management Device Market Report
- Global Implantable Cardiac Rhythm Management Device market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Implantable Cardiac Rhythm Management Device trade, costs, and supply chains
- Implantable Cardiac Rhythm Management Device market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Implantable Cardiac Rhythm Management Device market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Implantable Cardiac Rhythm Management Device market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Implantable Cardiac Rhythm Management Device supply chain analysis
- Implantable Cardiac Rhythm Management Device trade analysis, Implantable Cardiac Rhythm Management Device market price analysis, and Implantable Cardiac Rhythm Management Device supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Implantable Cardiac Rhythm Management Device market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Stryker Corporation
- Schiller AG
- Medtronic PLC
- Abbott Laboratories
- Boston Scientific Corporation
- Koninklijke Philips N.V.
- Zoll Medical Corporation
- Biotronik Ltd.
- MicroPort Scientific Corporation
- Abiomed Inc.
- Hydrix Limited
- Nihon Kohden Corporation
- Japan Lifeline Co. Ltd.
- Lepu Medical Technology Co. Ltd.
- LivaNova PLC
- Shenzhen Mindray Biomedical Electronics Co. Ltd.
- Progetti Srl
- Edwards Lifesciences Corporation
- Cardiac Science Corporation
- Sorin Group S.p.A.
- St. Jude Medical LLC
- Osprey Medical Inc.
- Bardy Diagnostics Inc.
- VivaLNK Inc.
- NanoMatriX International Limited
- Beijing Demax Medical Technology Co. Ltd.
- iRhythm Technologies Inc.
- Impulse Dynamics PLC
- SentiAR Inc.
- VytronUS Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
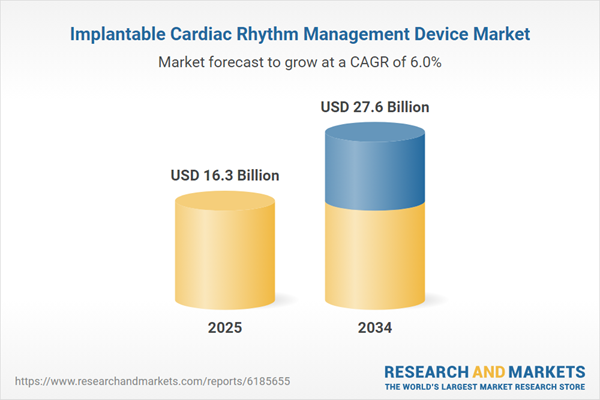
| Estimated Market Value ( USD | $ 16.3 Billion |
| Forecasted Market Value ( USD | $ 27.6 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |