The rituximab biosimilars market has emerged as a significant segment of the global biologics industry, offering an affordable alternative to the reference product, Rituxan, which is used in the treatment of various cancers and autoimmune diseases. Rituximab, a monoclonal antibody, has been widely prescribed for conditions such as non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), rheumatoid arthritis, and granulomatosis with polyangiitis. However, the high cost of the reference product has spurred the development of biosimilars, which are similar but not identical copies of the originator biologic. Rituximab biosimilars are designed to provide the same therapeutic effect, safety profile, and efficacy, while being more cost-effective for healthcare systems and patients. The growing focus on reducing healthcare expenditure and increasing access to biologic therapies in both developed and emerging markets has accelerated the uptake of rituximab biosimilars. As regulatory bodies, such as the FDA and EMA, continue to approve more biosimilars, the market for these drugs is expected to expand, further driving competition and innovation in biologic treatments.
The rituximab biosimilars market witnessed a surge in demand as healthcare providers and patients increasingly embraced these cost-effective alternatives. The growing pressure to control healthcare costs in both developed and emerging markets led to the widespread adoption of rituximab biosimilars in clinical practice. Additionally, several key biosimilar versions of rituximab received regulatory approval in major markets, further fueling competition and market growth. Companies such as Celltrion and Sandoz led the charge in launching their biosimilar products, positioning them as accessible options for patients requiring long-term treatment for autoimmune diseases and certain types of cancer. Furthermore, payers and insurers played a crucial role in promoting the use of biosimilars, providing financial incentives for physicians and hospitals to prescribe these more affordable alternatives. This shift towards biosimilars was also supported by positive clinical outcomes and increasing physician confidence in the safety and efficacy of rituximab biosimilars. Overall, 2024 marked a pivotal year for the market, as biosimilars became an integral part of treatment regimens for various conditions.
The rituximab biosimilars market is expected to experience further growth as more biosimilars enter the market and competition intensifies. With the expiration of patents for several reference biologics, including rituximab, the market will likely see an influx of new biosimilars developed by various global pharmaceutical companies. Additionally, advancements in biosimilar manufacturing processes and technology will lead to increased affordability, improving patient access to life-saving therapies. Regulatory bodies are expected to continue streamlining approval processes, providing faster market access for biosimilar developers. As healthcare systems increasingly prioritize cost-effective treatment options, biosimilars will play a critical role in reducing the financial burden on both patients and healthcare systems, especially in the oncology and autoimmune disease segments. The global shift towards biosimilars will also encourage greater collaboration between multinational pharmaceutical companies and local manufacturers, further expanding the reach of rituximab biosimilars into emerging markets. With expanding indications and increasing confidence in the safety and efficacy of these products, rituximab biosimilars are poised to become a cornerstone of treatment in the years to come.
Key Insights: Rituximab Biosimilars Market
- The growing acceptance of biosimilars in clinical settings, driven by positive clinical trial data and increasing physician confidence in their efficacy and safety.
- The expansion of rituximab biosimilars into new geographic markets, especially in emerging economies, where there is high demand for affordable biologic treatments.
- The development of new formulations and delivery methods for rituximab biosimilars to improve patient convenience and adherence to long-term treatment regimens.
- Increased investment in biosimilar R&D, leading to more competitive and affordable products entering the market.
- The growing role of payers and insurers in driving the adoption of biosimilars through reimbursement policies and cost-saving incentives.
- The rising cost of biologic therapies and the need for cost-effective alternatives to increase patient access to life-saving treatments.
- The expiration of patents for reference biologics like rituximab, leading to increased competition and market entry of biosimilars.
- Support from regulatory agencies, such as the FDA and EMA, which are approving more biosimilars and improving their market accessibility.
- The increasing prevalence of autoimmune diseases and cancer, which drives demand for long-term biologic therapies, creating a growing market for biosimilars.
- The primary challenge facing the rituximab biosimilars market is overcoming physician and patient hesitancy due to concerns over the long-term safety and efficacy of biosimilars compared to the reference biologic, despite the growing body of evidence supporting their use.
Rituximab Biosimilars Market Segmentation
By Route Of Administration
- Subcutaneous
- Intravenous
- Molecular Type
By Application
- Non-Hodgkin's Lymphoma
- Chronic Lymphocytic Leukemia
- Rheumatoid Arthritis
- Other Applications
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Mail Order
Key Companies Analysed
- Teva/Cellitron
- Novartis AG
- Pfizer
- BIOCAD
- Shanghai Henlius Biotech Inc.
- Sandoz
- Innovent Biologics Inc.
- Sinocelltech
- Cadila Pharmaceuticals
- Hetero Drugs Limited
- Dr. Reddy’s Laboratories
- Shanghai Fosun Pharmaceutical (Group) Co. Ltd.
- Reliance Life Sciences India
- Zenotech Laboratories
- NAPP Pharmaceuticals Limited
- Mundipharma Deutschland GmbH & Co. KG
- Celltrion
- Biocad
- MABION S.A.
- Janssen Pharmaceutical
- Amgen
- Teva Pharmaceuticals
- Celltrion Healthcare
- Coherus BioSciences
- Innovent Biologics
- Mylan
- Aryogen Biopharma
- TR-Pharm
- Hikma Pharmaceuticals plc.
Rituximab Biosimilars Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Rituximab Biosimilars Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Rituximab Biosimilars market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Rituximab Biosimilars market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Rituximab Biosimilars market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Rituximab Biosimilars market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Rituximab Biosimilars market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Rituximab Biosimilars value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Rituximab Biosimilars industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Rituximab Biosimilars Market Report
- Global Rituximab Biosimilars market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Rituximab Biosimilars trade, costs, and supply chains
- Rituximab Biosimilars market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Rituximab Biosimilars market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Rituximab Biosimilars market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Rituximab Biosimilars supply chain analysis
- Rituximab Biosimilars trade analysis, Rituximab Biosimilars market price analysis, and Rituximab Biosimilars supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Rituximab Biosimilars market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Teva/Cellitron
- Novartis AG
- Pfizer
- BIOCAD
- Shanghai Henlius Biotech Inc.
- Sandoz
- Innovent Biologics Inc.
- Sinocelltech
- Cadila Pharmaceuticals
- Hetero Drugs Limited
- Dr. Reddy’s Laboratories
- Shanghai Fosun Pharmaceutical (Group) Co. Ltd.
- Reliance Life Sciences India
- Zenotech Laboratories
- NAPP Pharmaceuticals Limited
- Mundipharma Deutschland GmbH & Co. KG
- Celltrion
- Biocad
- MABION S.A.
- Janssen Pharmaceutical
- Amgen
- Teva Pharmaceuticals
- Celltrion Healthcare
- Coherus BioSciences
- Innovent Biologics
- Mylan
- Aryogen Biopharma
- TR-Pharm
- Hikma Pharmaceuticals PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
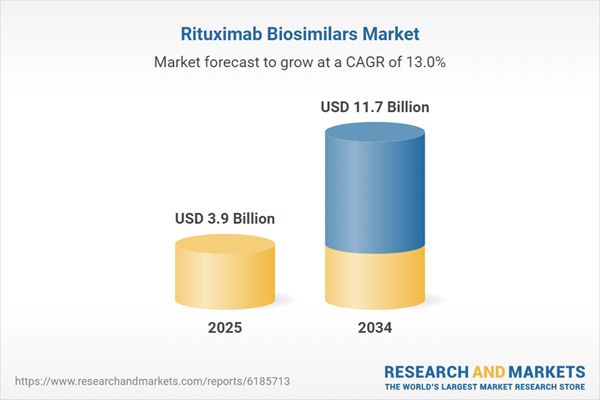
| Estimated Market Value ( USD | $ 3.9 Billion |
| Forecasted Market Value ( USD | $ 11.7 Billion |
| Compound Annual Growth Rate | 13.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |