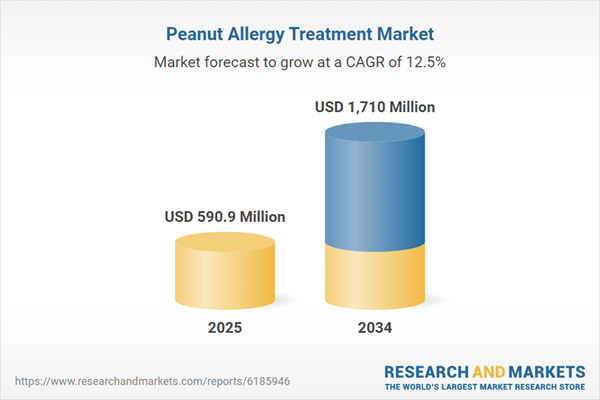
The global peanut allergy treatment market is expanding as the prevalence of peanut allergies continues to rise, particularly among children. Peanut allergies are among the most severe food allergies, often leading to life-threatening anaphylaxis, requiring immediate intervention. Traditionally, management of peanut allergies has relied on strict avoidance of peanut-containing foods and emergency epinephrine administration in case of accidental exposure. However, the emergence of immunotherapy-based treatments, such as oral immunotherapy (OIT) and biologic drugs, is transforming the treatment landscape. Leading companies such as Aimmune Therapeutics (Nestlé), DBV Technologies, and Regeneron Pharmaceuticals are investing in innovative solutions to desensitize allergic patients and reduce the severity of allergic reactions. Regulatory approvals for peanut allergy immunotherapies, combined with increasing research on long-term tolerance-building, are expected to drive market growth. As awareness of peanut allergy treatments increases and healthcare infrastructure improves, the demand for novel, effective therapies continues to rise.
The peanut allergy treatment market has witnessed key advancements, particularly in oral immunotherapy, biologic therapies, and diagnostic solutions. Palforzia, the first FDA-approved oral immunotherapy for peanut allergy, has expanded its market reach, with ongoing studies evaluating its long-term efficacy and safety in younger populations. Additionally, Viaskin Peanut, a skin-based immunotherapy patch developed by DBV Technologies, has gained traction as a needle-free alternative, especially for pediatric patients. Monoclonal antibody therapies, such as dupilumab and omalizumab, are being explored as potential treatments to modulate immune responses and prevent severe allergic reactions. Advances in predictive diagnostics and biomarker research have enabled improved patient selection for immunotherapy, enhancing treatment success rates. Meanwhile, regulatory agencies are increasingly emphasizing post-market surveillance to monitor the long-term safety and efficacy of emerging therapies. Despite these advancements, high treatment costs and accessibility barriers remain key challenges, particularly in low- and middle-income countries.
The peanut allergy treatment market is expected to see further developments in precision immunotherapy, gene-based therapies, and expanded access to desensitization programs. Research into gene-editing techniques, such as CRISPR-based approaches, is exploring the possibility of modifying immune responses at a genetic level to provide long-term allergy relief. Combination therapies integrating biologics with immunotherapy are likely to improve desensitization outcomes and reduce treatment duration. Additionally, advancements in food-based oral tolerance programs may lead to the development of novel therapeutics derived from modified peanut proteins with reduced allergenicity. Regulatory bodies and healthcare providers are expected to work toward increasing affordability and reimbursement policies for peanut allergy treatments, making them more accessible to a wider population. As innovation in allergy treatment accelerates, the focus will be on improving safety, long-term effectiveness, and patient adherence to therapies that provide sustained allergy protection.
Key Insights: Peanut Allergy Treatment Market
- Expansion of Oral Immunotherapy (OIT) Treatments: FDA-approved therapies such as Palforzia are gaining adoption, helping patients gradually build peanut tolerance and reduce severe allergic reactions.
- Development of Skin-Based Immunotherapy (Epicutaneous Therapy): Innovative allergy patches, such as Viaskin Peanut, offer a needle-free alternative for pediatric peanut allergy treatment.
- Advancements in Biologic Therapies for Immune Modulation: Monoclonal antibodies, including dupilumab and omalizumab, are being tested to prevent severe peanut allergy reactions and improve immune tolerance.
- Improved Peanut Allergy Diagnostic & Biomarker-Based Treatments: The use of biomarkers and predictive diagnostics is enabling personalized immunotherapy, improving treatment selection and success rates.
- Emergence of CRISPR & Gene-Editing Technologies for Allergy Management: Gene-based therapies are being explored as potential long-term solutions for peanut allergy by altering immune response pathways.
- Rising Prevalence of Peanut Allergies, Especially in Children: Increasing cases of peanut allergies globally are driving demand for new, effective treatment options beyond avoidance strategies.
- Regulatory Approvals & Advancements in Immunotherapy: Growing regulatory support for peanut allergy immunotherapies is expanding patient access to FDA- and EMA-approved treatments.
- Increased Research in Precision Medicine & Allergy Tolerance Development: Ongoing studies in personalized immunotherapy and long-term allergy tolerance are enhancing treatment efficacy and safety.
- Growing Consumer Awareness & Healthcare Infrastructure Expansion: Improved awareness and healthcare accessibility are encouraging early diagnosis and treatment adoption for peanut allergy patients.
- High Treatment Costs & Limited Access to Immunotherapy: The high price of peanut allergy immunotherapies and the lack of reimbursement in many regions limit affordability and accessibility for a large portion of patients.
Peanut Allergy Treatment Market Segmentation
By Drug Class
- Antihistamines
- Immunotherapies
- Epinephrine
- Other Drug Classes
By Route Of Administration
- Injectable
- Oral
- Other Routes Of Administration
By Distribution Channel
- Retail Pharmacy
- Hospital Pharmacy
- Other Distribution Channels
Key Companies Analysed
- Pfizer Inc.
- F. Hoffmann-La Roche AG
- Sanofi SA
- Novartis AG
- Takeda Pharmaceutical Company Limited
- Viatris Inc.
- Regeneron Pharmaceuticals Inc.
- Hikma Pharmaceuticals plc
- ALK-Abelló A/S
- Amneal Pharmaceuticals Inc.
- Amphastar Pharmaceuticals Inc
- Stallergenes Greer plc
- kaleo Inc.
- Aquestive Therapeutics Inc.
- Vedanta Biosciences Inc.
- Aimmune Therapeutics Inc.
- AnaptysBio Inc.
- Alladapt Immunotherapeutics Inc.
- Aravax Pty Ltd
- Intrommune Therapeutics Inc.
- IgGenix Inc.
- DBV Technologies SA
- Camallergy Ltd.
- HAL Allergy Group
- Prota Therapeutics Pty Ltd.
Peanut Allergy Treatment Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Peanut Allergy Treatment Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Peanut Allergy Treatment market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Peanut Allergy Treatment market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Peanut Allergy Treatment market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Peanut Allergy Treatment market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Peanut Allergy Treatment market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Peanut Allergy Treatment value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Peanut Allergy Treatment industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Peanut Allergy Treatment Market Report
- Global Peanut Allergy Treatment market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Peanut Allergy Treatment trade, costs, and supply chains
- Peanut Allergy Treatment market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Peanut Allergy Treatment market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Peanut Allergy Treatment market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Peanut Allergy Treatment supply chain analysis
- Peanut Allergy Treatment trade analysis, Peanut Allergy Treatment market price analysis, and Peanut Allergy Treatment supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Peanut Allergy Treatment market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- F. Hoffmann-La Roche AG
- Sanofi SA
- Novartis AG
- Takeda Pharmaceutical Company Limited
- Viatris Inc.
- Regeneron Pharmaceuticals Inc.
- Hikma Pharmaceuticals PLC
- ALK-Abelló A/S
- Amneal Pharmaceuticals Inc.
- Amphastar Pharmaceuticals Inc.
- Stallergenes Greer PLC
- kaleo Inc.
- Aquestive Therapeutics Inc.
- Vedanta Biosciences Inc.
- Aimmune Therapeutics Inc.
- AnaptysBio Inc.
- Alladapt Immunotherapeutics Inc.
- Aravax Pty Ltd.
- Intrommune Therapeutics Inc.
- IgGenix Inc.
- DBV Technologies SA
- Camallergy Ltd.
- HAL Allergy Group
- Prota Therapeutics Pty Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 590.9 Million |
| Forecasted Market Value ( USD | $ 1710 Million |
| Compound Annual Growth Rate | 12.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |