The small molecule injectable drugs market represents a crucial segment of modern pharmaceutical care, offering rapid, effective drug delivery for a wide range of acute and chronic conditions. These drugs are typically administered intravenously, intramuscularly, or subcutaneously, allowing for fast onset of action, bypassing first-pass metabolism, and achieving precise dosage control. Their use spans across therapeutic areas such as oncology, infectious diseases, cardiovascular disorders, and autoimmune conditions. Unlike oral drugs, injectables are often employed when immediate therapeutic impact is required or when patient conditions preclude oral administration. This market is supported by both branded and generic manufacturers, with significant contributions from hospital formularies and ambulatory settings. Rising prevalence of lifestyle-related diseases, increased demand for critical care treatments, and the expansion of biologic-drug competition through injectable small molecule therapies have all driven growth. As healthcare infrastructure improves in emerging economies and injectable drug formulations become more patient-friendly, including the advent of self-administrable autoinjectors and prefilled syringes, the market is seeing rising adoption across a broader demographic.
The small molecule injectable drugs market experienced a notable surge in innovation, regulatory activity, and capacity expansion. Demand remained especially strong in oncology and anti-infective classes, where injectable formulations continue to dominate treatment protocols. Manufacturers prioritized the development of ready-to-use (RTU) injectables, minimizing preparation time and reducing the risk of contamination in clinical environments. Additionally, CDMOs specializing in sterile injectables reported increased collaborations with pharmaceutical companies seeking to outsource both formulation development and fill-finish operations. Regulatory agencies emphasized the need for drug product stability, particularly for high-potency APIs and complex injectable formulations, leading to more investments in lyophilization and dual-chamber systems. In parallel, the generics industry made strides by launching injectable versions of off-patent small molecule blockbusters, lowering costs for health systems and patients alike. Digital traceability and serialization requirements were also expanded globally, requiring firms to integrate track-and-trace solutions to ensure security and compliance in injectable drug distribution.
The small molecule injectable drugs market is expected to benefit from technological integration, novel drug-device combinations, and expanding therapeutic pipelines. Advances in formulation science will support the development of long-acting injectables and depot-based systems, enabling sustained drug release over days or weeks, thus improving adherence and convenience. As personalized medicine continues to gain momentum, injectable drugs with tailored dosing regimens for specific patient profiles will gain favor, particularly in oncology and rare diseases. Biopharma companies are anticipated to increase focus on subcutaneous delivery systems, offering more home-friendly alternatives to intravenous infusions. Simultaneously, global health initiatives are likely to drive access to essential injectable medicines in underserved regions, necessitating innovations in cold-chain logistics and cost-effective packaging. Moreover, with more small molecule drugs receiving fast-track designations and orphan drug status, rapid injectable formulation development will remain a strategic priority. Companies that invest in flexible manufacturing platforms, digital validation, and regulatory agility will be well-positioned to lead in this evolving landscape.
Key Insights: Small Molecule Injectable Drugs Market
- Growth in Ready-to-Use (RTU) Formulations: RTU injectable drugs are gaining popularity as they improve safety, minimize dosage errors, and streamline workflows in clinical and hospital settings, particularly in emergency and oncology care.
- Expansion of Prefilled Syringes and Autoinjectors: With a focus on self-administration and outpatient care, pharmaceutical companies are increasingly launching small molecule drugs in user-friendly prefilled delivery systems to enhance patient convenience and adherence.
- Rise in Outsourcing to Sterile Injectable CDMOs: Drug developers are leveraging the specialized infrastructure of CDMOs to meet growing demand for sterile injectable manufacturing, reducing capital expenditures and accelerating time-to-market.
- Advancement in Long-Acting Injectables: The development of depot formulations and extended-release injectables is enabling once-weekly or once-monthly dosing schedules, offering improved outcomes in psychiatric, hormonal, and chronic disease treatment.
- Focus on Digital Serialization and Track-and-Trace: Regulatory pressure is driving manufacturers to implement robust serialization and digital monitoring systems to ensure supply chain integrity and reduce counterfeit risks for injectable drugs.
- Increasing Prevalence of Chronic and Acute Diseases: Rising global incidence of conditions requiring rapid or targeted drug delivery - such as cancer, infections, and autoimmune diseases - is fueling consistent demand for injectable treatments.
- Favorable Regulatory Support for Generics and Biosimilars: Streamlined approval processes and patent expirations are encouraging the entry of generic injectable drugs, increasing affordability and boosting access in both developed and emerging markets.
- Advancements in Drug Delivery Technologies: Innovations in formulation and device integration are expanding the range of injectable options, enabling new therapeutic uses and improved patient experiences in clinical and home settings.
- Healthcare Infrastructure Expansion in Emerging Markets: Improvements in hospital networks, cold-chain capabilities, and funding for essential medicines are accelerating injectable drug adoption in underserved regions worldwide.
- Complexity of Sterile Manufacturing: Injectable drugs require strict aseptic conditions and advanced infrastructure, making manufacturing costly and technically demanding. Quality lapses can lead to recalls or regulatory penalties, creating barriers for new entrants and increasing operational risks for manufacturers.
Small Molecule Injectable Drugs Market Segmentation
By Drug Class
- Small Molecule Antibiotics
- Analgesics
- Chemotherapy
- Antivirals
- Anticoagulant
- Skeletal Muscle Relaxants
- Anticonvulsants
- Other Drug Class
By Indication
- Pain Management
- Oncology
- Infectious Diseases
- Cardiovascular Diseases
- CNS Diseases
- Other Indications
By Mode of Delivery
- IV Set
- Intravenous Injection
- Infusion Pump
- Intramuscular Injection
- Subcutaneous Injection
By End User
- Hospitals
- Ambulatory Clinics
- Outpatient Facility
- Infusion Therapy Center
- Home Care
- Other End Users
Key Companies Analysed
- Pfizer Inc.
- Johnson & Johnson Services Inc.
- F. Hoffmann-La Roche AG
- Merck & Co. Inc.
- AbbVie Inc.
- Sanofi S.A.
- Bristol-Myers Squibb Company
- AstraZeneca plc
- Abbott Laboratories
- Novartis AG
- Fresenius Kabi AG
- GlaxoSmithKline plc
- Eli Lilly and Company
- Gilead Sciences Inc.
- Amgen Inc.
- Becton Dickinson and Company
- Teva Pharmaceutical Industries Ltd.
- Baxter International Inc.
- Sun Pharmaceutical Industries Ltd.
- Aurobindo Pharma Ltd.
- Dr. Reddy’s Laboratories Ltd.
- Cipla Limited
- Hikma Pharmaceuticals plc
- BioCryst Pharmaceuticals Inc.
- Mylan N.V.
Small Molecule Injectable Drugs Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Small Molecule Injectable Drugs Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Small Molecule Injectable Drugs market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Small Molecule Injectable Drugs market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Small Molecule Injectable Drugs market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Small Molecule Injectable Drugs market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Small Molecule Injectable Drugs market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Small Molecule Injectable Drugs value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Small Molecule Injectable Drugs industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Small Molecule Injectable Drugs Market Report
- Global Small Molecule Injectable Drugs market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Small Molecule Injectable Drugs trade, costs, and supply chains
- Small Molecule Injectable Drugs market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Small Molecule Injectable Drugs market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Small Molecule Injectable Drugs market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Small Molecule Injectable Drugs supply chain analysis
- Small Molecule Injectable Drugs trade analysis, Small Molecule Injectable Drugs market price analysis, and Small Molecule Injectable Drugs supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Small Molecule Injectable Drugs market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Johnson & Johnson Services Inc.
- F. Hoffmann-La Roche AG
- Merck & Co. Inc.
- AbbVie Inc.
- Sanofi S.A.
- Bristol-Myers Squibb Company
- AstraZeneca PLC
- Abbott Laboratories
- Novartis AG
- Fresenius Kabi AG
- GlaxoSmithKline PLC
- Eli Lilly and Company
- Gilead Sciences Inc.
- Amgen Inc.
- Becton Dickinson and Company
- Teva Pharmaceutical Industries Ltd.
- Baxter International Inc.
- Sun Pharmaceutical Industries Ltd.
- Aurobindo Pharma Ltd.
- Dr. Reddy’s Laboratories Ltd.
- Cipla Limited
- Hikma Pharmaceuticals PLC
- BioCryst Pharmaceuticals Inc.
- Mylan N.V.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
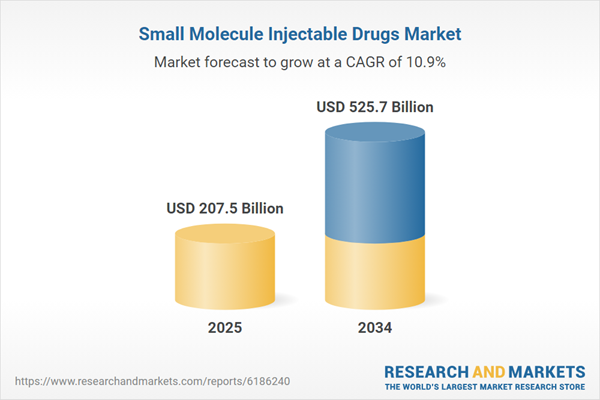
| Estimated Market Value ( USD | $ 207.5 Billion |
| Forecasted Market Value ( USD | $ 525.7 Billion |
| Compound Annual Growth Rate | 10.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |