The Interventional Neurology Devices and Equipment Market encompasses minimally invasive tools and systems used to diagnose and treat disorders of the brain, spine, and surrounding vasculature. This includes neurovascular catheters, stent retrievers, embolic coils, thrombectomy devices, and balloon occlusion systems, primarily used in procedures for stroke, aneurysms, arteriovenous malformations (AVMs), and intracranial stenosis. With neurological diseases being a leading cause of disability and death worldwide - particularly ischemic stroke - the demand for rapid and targeted interventions is growing. These devices enable real-time image-guided access to delicate neural structures with lower risk and faster recovery than traditional open surgery. As stroke treatment windows narrow and clinical guidelines evolve to favor mechanical thrombectomy, the market is seeing greater investment from healthcare systems and manufacturers alike. The shift toward precision neurology and expanding interventional capabilities is positioning this market as a cornerstone of next-generation neurocare.
The interventional neurology market continued to expand with increased adoption of mechanical thrombectomy for acute ischemic stroke, supported by updated clinical guidelines and wider hospital access. Leading companies such as Stryker, Penumbra, and Medtronic introduced enhanced neurovascular access systems with improved flexibility and deliverability. Stent retrievers and aspiration catheters saw broader use across both primary and secondary stroke centers, while combination therapies - pairing thrombolytics with device-based intervention - became more common. Artificial intelligence began playing a larger role in stroke triage and imaging analysis, helping to reduce door-to-treatment time. Robotic navigation systems were introduced in a limited number of centers to assist with intracranial stenting and aneurysm coiling procedures. Government investments in stroke-ready hospital networks, especially across Asia-Pacific and Latin America, supported adoption of mobile stroke units and portable imaging tools integrated with interventional neurology kits. Meanwhile, neurosurgeons and interventional radiologists collaborated more closely to expand indications for endovascular neurointerventions.
The interventional neurology market is set to evolve with more intelligent, adaptive devices and expanded therapeutic applications. Smart catheters embedded with sensors will monitor vessel dynamics during intervention, alerting operators in real time to complications or changes in blood flow. AI-powered imaging tools will enhance precision during aneurysm coiling and AVM embolization, while next-generation clot retrievers will improve efficacy in smaller or more distal vessels. Robotics will become more widely used for fine motor control in deep brain and spinal navigation. Moreover, broader training programs and simulation platforms will be introduced to upskill neuro-interventionalists in emerging regions. As the burden of neurovascular diseases continues to rise, especially in aging populations, healthcare systems will prioritize early intervention capabilities and invest in regional stroke centers with full interventional support. These shifts will reinforce interventional neurology’s role as a critical, high-growth frontier in neurovascular care.
Key Insights: Interventional Neurology Devices and Equipment Market
- The analyst observes increasing adoption of AI-enhanced stroke triage and imaging analysis tools that support faster diagnosis, patient selection, and treatment decision-making in emergency neurovascular care.
- Smart neurovascular devices with embedded sensors are trending, enabling real-time feedback during procedures and helping improve outcomes through intraoperative monitoring and complication avoidance, says the analyst.
- According to the analyst, robotic-assisted navigation is gaining ground in complex brain interventions, where precision and reduced hand tremor are critical in coiling, stenting, and embolization procedures.
- The analyst highlights the expansion of mobile stroke units and tele-intervention models, enabling remote guidance of emergency neurointerventions in rural and underserved areas.
- Comprehensive stroke centers are investing in hybrid operating rooms and digital neurointervention platforms that support multi-modal imaging and advanced neuro-device compatibility, notes the analyst.
- The analyst identifies rising incidence of ischemic strokes and neurological emergencies, particularly in aging populations, as a primary driver for expanding interventional neurology capabilities and device demand.
- Guideline updates recommending mechanical thrombectomy for a broader patient group are increasing procedural volumes and encouraging hospitals to invest in interventional neurology infrastructure, says the analyst.
- The analyst notes that improved device technologies - such as next-gen stent retrievers and aspiration systems - are reducing complications and expanding eligibility for neurointervention in complex cases.
- Healthcare digitization and AI-based imaging support are accelerating diagnosis-to-treatment timelines, enhancing overall stroke recovery outcomes and driving investment in neuro-interventional platforms, according to the analyst.
- The analyst highlights the shortage of trained interventional neurologists and neurosurgeons, especially in low-resource regions, as a key barrier to expanding access to timely neurovascular procedures.
- According to the analyst, high costs associated with neurointerventional equipment, imaging systems, and procedural infrastructure limit adoption in community hospitals and rural stroke centers.
Interventional Neurology Devices and Equipment Market Segmentation
By Type
- Aneurysm Coiling & Embolization Devices
- Cerebrospinal Fluid Management Devices
- Neurothrombectomy Devices
- Support Devices)
- Aneurysm Coiling & Embolization Devices By Type
- Embolic coils
- Flow diversion devices
- Liquid embolic devices)
- Angioplasty Devices by Type
- Carotid artery stents
- Embolic protection systems)
- Support Devices By Type
- Micro guide wires
- Micro catheters)
- Neurothrombectomy Devices By Type
- CLOT retrieval devices
- Suction and aspiration devices
- Snare
By End-User
- Hospitals
- Neurology clinics
- Ambulatory care centers and others
Key Companies Analysed
- Medtronic plc
- Stryker Corporation
- Terumo Corporation
- Penumbra Inc.
- COVIDien
- Abbott Laboratories
- Merit Medical Systems Inc.
- W. L. Gore & Associates
- Bayer AG
- MicroPort Scientific Corporation
- Boston Scientific Corporation
- Johnson & Johnson
- Medikit Co. Ltd.
- Acandis GmbH
- Biosensors International Ltd.
- Integra LifeSciences Holdings Corporation
- Magstim C-Ltd.
- UreSil LLC
- Cook Group Incorporated
- Siemens Healthineers
- B. Braun Melsungen AG
- Integer Holdings Corporation
- Nihon Kohden Corporation
- LivaNova
- Teleflex Incorporated
- Balt Extrusion SAS
- Rapid Medical Ltd.
- phenox GmbH
- Spiegelberg GmbH & Co. KG
Interventional Neurology Devices and Equipment Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Interventional Neurology Devices and Equipment Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Interventional Neurology Devices and Equipment market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Interventional Neurology Devices and Equipment market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Interventional Neurology Devices and Equipment market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Interventional Neurology Devices and Equipment market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Interventional Neurology Devices and Equipment market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Interventional Neurology Devices and Equipment value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Interventional Neurology Devices and Equipment industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Interventional Neurology Devices and Equipment Market Report
- Global Interventional Neurology Devices and Equipment market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Interventional Neurology Devices and Equipment trade, costs, and supply chains
- Interventional Neurology Devices and Equipment market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Interventional Neurology Devices and Equipment market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Interventional Neurology Devices and Equipment market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Interventional Neurology Devices and Equipment supply chain analysis
- Interventional Neurology Devices and Equipment trade analysis, Interventional Neurology Devices and Equipment market price analysis, and Interventional Neurology Devices and Equipment supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Interventional Neurology Devices and Equipment market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Medtronic PLC
- Stryker Corporation
- Terumo Corporation
- Penumbra Inc.
- COVIDien
- Abbott Laboratories
- Merit Medical Systems Inc.
- W. L. Gore & Associates
- Bayer AG
- MicroPort Scientific Corporation
- Boston Scientific Corporation
- Johnson & Johnson
- Medikit Co. Ltd.
- Acandis GmbH
- Biosensors International Ltd.
- Integra LifeSciences Holdings Corporation
- Magstim C-Ltd.
- UreSil LLC
- Cook Group Incorporated
- Siemens Healthineers
- B. Braun Melsungen AG
- Integer Holdings Corporation
- Nihon Kohden Corporation
- LivaNova
- Teleflex Incorporated
- Balt Extrusion SAS
- Rapid Medical Ltd.
- phenox GmbH
- Spiegelberg GmbH & Co. KG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
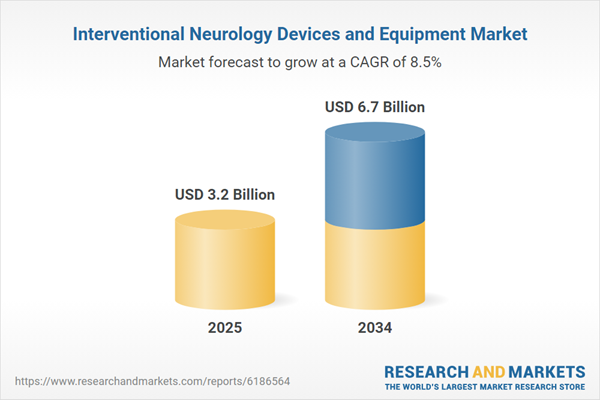
| Estimated Market Value ( USD | $ 3.2 Billion |
| Forecasted Market Value ( USD | $ 6.7 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |