The Intranasal Drug Delivery Devices Market offers a non-invasive, rapid, and patient-friendly method for delivering therapeutic agents through the nasal mucosa. This route is especially valuable for delivering drugs that require fast systemic absorption or direct access to the central nervous system, bypassing the blood-brain barrier. Applications include pain management, seizure rescue, hormone therapy, allergy relief, and more recently, vaccines and psychiatric medications. Devices range from metered-dose sprays to soft mist inhalers and nasal powder dispensers, often featuring precision dosing and portability. With rising demand for self-administered, needle-free therapies and increasing awareness of patient compliance challenges, intranasal delivery is gaining favor across both emergency and chronic care settings. As drug developers formulate molecules tailored for nasal absorption, device manufacturers are innovating to optimize drug deposition, reduce irritation, and enhance bioavailability, especially for pediatric, geriatric, and needle-phobic populations.
The market saw robust activity, particularly in emergency and central nervous system applications. FDA approvals and market expansions for intranasal naloxone and benzodiazepine rescue therapies drove clinical adoption. Devices with user-friendly designs and intuitive mechanisms gained popularity, with pharma-device collaborations streamlining co-development. Aptar Pharma and Kurve Technology released advanced intranasal platforms supporting tight dose control and directional delivery for CNS-targeted agents. The intranasal delivery route was also explored for COVID-19 boosters and flu vaccines, prompting increased investment in mucosal immunology. Additionally, allergy treatment and hormone therapies, including intranasal oxytocin and calcitonin, were reformulated into more stable, travel-friendly nasal sprays. Remote monitoring and smart devices capable of tracking usage were piloted in clinical trials. With growing recognition of the intranasal route’s speed, safety, and accessibility, regulatory bodies showed increased receptiveness to fast-track reviews of innovative formulations.
The intranasal drug delivery devices market is expected to grow significantly with the development of biologic and peptide-based drugs for nasal administration. Innovations will include thermosensitive gels and mucoadhesive systems that prolong nasal residence time and improve absorption. Nasal vaccines for respiratory viruses and neurological disorders will enter later-stage trials, with intranasal platforms playing a pivotal role in pandemic preparedness strategies. Device personalization - based on nasal cavity anatomy, age, or flow rate - will enhance user experience and improve clinical outcomes. Integration of AI for dose timing, adherence tracking, and remote patient support will expand, particularly in behavioral and pain management therapies. Regulatory harmonization across markets will allow global launches of advanced combination products. As the healthcare system shifts toward decentralization and home-based care, intranasal delivery will offer a safe, effective, and scalable option for multiple therapeutic areas, anchoring its long-term market potential.
Key Insights: Intranasal Drug Delivery Devices Market
- The analyst highlights the rise of intranasal therapies for emergency care, particularly for opioid overdose reversal and seizure management, where speed and ease of use are critical in out-of-hospital settings.
- Smart intranasal delivery devices with digital adherence tracking and feedback features are trending, enabling better patient engagement and physician oversight in chronic therapies, especially for hormonal and psychiatric conditions.
- According to the analyst, intranasal vaccines for flu, COVID-19, and RSV are advancing in development pipelines, driven by demand for mucosal immunity and non-injectable delivery in global immunization campaigns.
- The analyst observes increasing investment in formulations for CNS drugs delivered via the nasal route, leveraging its ability to bypass the blood-brain barrier and rapidly deliver active compounds to brain tissues.
- Personalized intranasal device design based on patient-specific anatomy and flow dynamics is emerging, aiming to improve drug deposition and treatment efficacy across age groups and clinical conditions.
- The analyst identifies growing preference for non-invasive, self-administered therapies as a major driver, particularly among patients seeking convenient alternatives to injections or oral medications in chronic and acute conditions.
- Rising incidence of opioid overdoses and epilepsy-related emergencies is boosting demand for rapid-onset intranasal rescue medications that can be administered safely by laypersons, according to the analyst.
- The analyst notes that technological innovation in nasal spray devices - such as dose metering, anti-clog features, and breath-actuation - is enhancing usability and clinical confidence in home-based care settings.
- Increased research into mucosal immunity and central nervous system drug delivery is supporting broader pharmaceutical interest in developing nasal formulations for previously injection-only biologics and peptides, says the analyst.
- The analyst points out that variability in nasal anatomy, mucosal health, and patient technique can affect drug absorption and bioavailability, complicating standardization and requiring patient education or device customization.
- According to the analyst, formulation stability - especially for peptides and biologics - remains a challenge, with many compounds susceptible to degradation or requiring complex carrier systems for intranasal delivery.
Intranasal Drug Delivery Devices Market Segmentation
By Product
- Powder Delivery Device
- Liquid Delivery Device
- Pressurized Metered-Dose Inhalers
- Other Products
By Dosage
- Unit-Dose
- Multi-Dose
By Application
- Chronic Obstructive Pulmonary Disease (COPD)
- Rhinitis
- Cystic Fibrosis
- Nasal Congestion
- Asthma
- Other Applications
By End User
- Hospitals
- Clinics
- Homecare
- Other End Users
Key Companies Analysed
- Johnson and Johnson Inc.
- Merck and Co.Inc.
- Novartis AG
- AstraZeneca plc.
- GlaxoSmithKline Plc
- 3M Company
- Becton
- Dickinson and Company
- Teva Pharmaceutical Industries Ltd.
- AptarGroup Inc.
- West Pharmaceutical Services Inc.
- Teleflex Incorporated
- Recipharm AB
- Nemera Development S.A.
- Consort Medical plc.
- Vectura Group plc
- Douglas Pharmaceuticals Ltd.
- Avanir Pharmaceuticals
- OptiNoset Inc.
- Flamel Technologies
- Aegis Therapeutics LLC
- Sonoma Pharmaceuticals
- PendoPharm Inc.
- Kurve Technology Inc.
- Impel NeuroPharma
- Impexium Medical Products
- B.F. Ascher and Company Inc.
- ENT Technologies Pty. Ltd.
- Neurelis Inc.
- Generex Biotechnology Corporation
Intranasal Drug Delivery Devices Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Intranasal Drug Delivery Devices Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Intranasal Drug Delivery Devices market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Intranasal Drug Delivery Devices market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Intranasal Drug Delivery Devices market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Intranasal Drug Delivery Devices market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Intranasal Drug Delivery Devices market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Intranasal Drug Delivery Devices value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Intranasal Drug Delivery Devices industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Intranasal Drug Delivery Devices Market Report
- Global Intranasal Drug Delivery Devices market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Intranasal Drug Delivery Devices trade, costs, and supply chains
- Intranasal Drug Delivery Devices market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Intranasal Drug Delivery Devices market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Intranasal Drug Delivery Devices market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Intranasal Drug Delivery Devices supply chain analysis
- Intranasal Drug Delivery Devices trade analysis, Intranasal Drug Delivery Devices market price analysis, and Intranasal Drug Delivery Devices supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Intranasal Drug Delivery Devices market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Johnson and Johnson Inc.
- Merck and Co.Inc.
- Novartis AG
- AstraZeneca PLC
- GlaxoSmithKline PLC
- 3M Company
- Becton
- Dickinson and Company
- Teva Pharmaceutical Industries Ltd.
- AptarGroup Inc.
- West Pharmaceutical Services Inc.
- Teleflex Incorporated
- Recipharm AB
- Nemera Development S.A.
- Consort Medical PLC
- Vectura Group PLC
- Douglas Pharmaceuticals Ltd.
- Avanir Pharmaceuticals
- OptiNoset Inc.
- Flamel Technologies
- Aegis Therapeutics LLC
- Sonoma Pharmaceuticals
- PendoPharm Inc.
- Kurve Technology Inc.
- Impel NeuroPharma
- Impexium Medical Products
- B.F. Ascher and Company Inc.
- ENT Technologies Pty. Ltd.
- Neurelis Inc.
- Generex Biotechnology Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
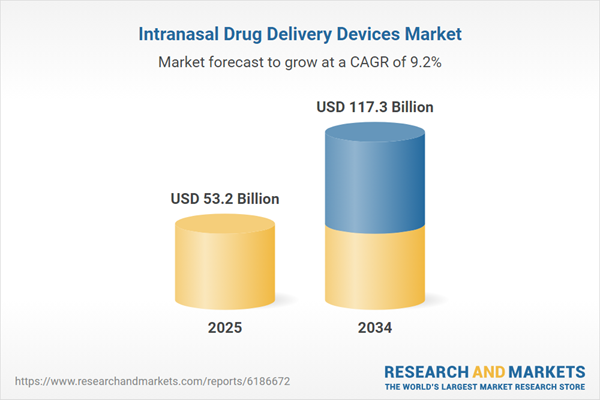
| Estimated Market Value ( USD | $ 53.2 Billion |
| Forecasted Market Value ( USD | $ 117.3 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |