Market Overview: Endometrial Ablation Devices Market
The endometrial ablation devices market has witnessed significant growth due to the increasing prevalence of abnormal uterine bleeding (AUB) and the rising demand for minimally invasive gynecological procedures. Endometrial ablation is an effective treatment option for women suffering from heavy menstrual bleeding, offering an alternative to hysterectomy with reduced recovery time and fewer complications. The growing preference for outpatient procedures and advancements in ablation techniques, such as radiofrequency, cryoablation, and microwave energy, have enhanced treatment outcomes and patient satisfaction. Moreover, the increasing awareness about women’s reproductive health and the availability of innovative devices have expanded the adoption of endometrial ablation across healthcare facilities. The market’s expansion is also driven by technological innovations that improve precision, safety, and ease of use, making these devices more accessible for gynecologists worldwide. As healthcare providers focus on reducing hospital stays and optimizing treatment costs, the demand for advanced ablation devices continues to rise, positioning the market for sustained growth.The endometrial ablation devices market experienced notable advancements, primarily fueled by the integration of artificial intelligence (AI) and real-time imaging technologies. AI-assisted procedural planning and automated ablation techniques enhanced precision, reducing the risk of complications and improving clinical efficiency. The growing availability of portable and office-based ablation devices contributed to the widespread adoption of in-office procedures, eliminating the need for hospitalization and reducing overall healthcare costs. Additionally, increasing regulatory approvals for next-generation ablation devices with enhanced safety features and better thermal control mechanisms expanded market opportunities. The year also saw an increase in awareness campaigns and educational programs focusing on minimally invasive gynecological treatments, encouraging more women to opt for endometrial ablation as a first-line therapy. As reimbursement policies for outpatient ablation procedures improved in several regions, accessibility and affordability further boosted the market’s growth. The continued expansion of women’s health clinics and ambulatory surgical centers played a crucial role in increasing procedural volumes.
The market is expected to undergo transformative developments driven by advancements in robotic-assisted ablation systems and the growing integration of telemedicine for pre- and post-procedure consultations. The emergence of smart ablation devices equipped with real-time monitoring and feedback mechanisms will further enhance procedural accuracy and safety. Additionally, the increasing shift toward personalized medicine will lead to the development of customizable ablation therapies tailored to individual patient needs. The adoption of biodegradable and bioresorbable ablation materials is anticipated to reduce long-term complications and improve patient outcomes. Emerging economies will become key growth drivers, with governments investing in expanding access to women’s healthcare services and modernizing medical infrastructure. Furthermore, collaborations between medical device companies and research institutions will accelerate the development of next-generation endometrial ablation technologies. As healthcare systems continue to prioritize minimally invasive treatments and enhanced patient care, the endometrial ablation devices market is set to experience steady growth, supported by continuous innovation and strategic market expansion.
Key Insights: Endometrial Ablation Devices Market
- AI-Driven Ablation Technologies: The integration of artificial intelligence in ablation devices is improving procedural precision by enabling automated energy delivery and real-time adjustments. AI-driven analytics enhance treatment planning, reduce procedural errors, and ensure optimal ablation coverage, leading to improved patient outcomes.
- Growth of Office-Based and Outpatient Procedures: The increasing availability of portable and easy-to-use ablation devices has fueled the shift toward office-based procedures. This trend reduces hospitalization costs, minimizes downtime for patients, and enhances accessibility to treatment, particularly in outpatient gynecology clinics.
- Expansion of Smart Ablation Devices with Real-Time Monitoring: The demand for smart ablation technologies featuring thermal monitoring, impedance tracking, and real-time feedback systems is rising. These innovations enhance safety, prevent excessive tissue damage, and improve procedural efficiency, ensuring better patient care.
- Development of Biodegradable Ablation Materials: Research in bioresorbable materials is paving the way for next-generation ablation technologies that minimize post-procedure complications. These materials help reduce inflammation, lower the risk of adhesions, and improve long-term treatment outcomes for patients undergoing endometrial ablation.
- Rising Adoption of Telemedicine for Pre- and Post-Procedure Consultations: With the increasing digitalization of healthcare, telemedicine is playing a key role in patient consultations, follow-ups, and post-procedure monitoring. Virtual healthcare platforms enable seamless patient-doctor communication, improving compliance and treatment effectiveness.
- Rising Prevalence of Abnormal Uterine Bleeding (AUB): The growing number of women suffering from AUB and related gynecological disorders is fueling demand for endometrial ablation procedures. Minimally invasive treatments are becoming the preferred choice over hysterectomy, leading to higher adoption rates.
- Technological Advancements in Ablation Devices: Innovations such as microwave, cryoablation, and radiofrequency technologies have enhanced procedural efficiency and patient safety. Continuous improvements in ablation techniques are enabling better treatment customization and reducing procedure time.
- Increasing Preference for Minimally Invasive Procedures: The shift toward minimally invasive treatments is driving the demand for advanced ablation devices. Patients prefer procedures with shorter recovery periods, minimal post-operative pain, and reduced hospital stays, making endometrial ablation an attractive option.
- Government and Private Sector Investments in Women’s Health: Rising healthcare expenditure and targeted investments in women’s health services are expanding access to gynecological treatments. Improved awareness and funding initiatives are driving market growth, particularly in developing regions.
- Regulatory Hurdles and Safety Concerns: Stringent regulatory requirements for medical device approvals and concerns regarding procedural risks such as uterine perforation and post-ablation syndrome pose challenges to market expansion. Compliance with evolving safety standards and obtaining necessary certifications can delay product launches and limit market penetration.
Endometrial Ablation Devices Market Segmentation
By Device Type
- Hysteroscopy Devices
- Thermal Balloon Ablators
- Radiofrequency Endometrial Ablation Devices
- Hydrothermal Ablators
- Electrical Ablators
- Other Device Types
By Technology
- Radiofrequency Ablation
- Cryoablation
- Hydrothermal Ablation
- Thermal Balloon
- Hysteroscopic Ablation
- Other Technologies
By End User
- Ambulatory Surgery Center
- Clinic
- Hospital
Key Companies Analysed
- Johnson and Johnson Private Limited
- Medtronic Plc.
- Stryker Corporation
- Boston Scientific Corporation
- Olympus Corporation
- Smith and Nephew plc.
- Hologic Inc.
- C R.Bard Inc.
- Cooper Companies Inc.
- Cook Medical
- Karl Storz GmbH and Co. KG
- Biotronik SE and Co. KG
- B. Braun Medical Inc.
- Richard Wolf GmbH
- Aesculap Inc.
- AngioDynamics
- Gyrus ACMI Inc.
- Minerva Surgical Inc.
- Minerva Surgical Inc.
- Lina Medical ApS
- Biolitec AG
- AEGEA Medical Inc.
- Albyn Medical
- Channel Medsystems Inc.
- CathRX Ltd
- Ecleris Srl.
- Idoman Teoranta
- Surkon Medical Co. Ltd
- Omnitech Systems Inc.
- Veldana Medical SA.
Endometrial Ablation Devices Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Endometrial Ablation Devices Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Endometrial Ablation Devices market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Endometrial Ablation Devices market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Endometrial Ablation Devices market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Endometrial Ablation Devices market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Endometrial Ablation Devices market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Endometrial Ablation Devices value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Endometrial Ablation Devices industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Endometrial Ablation Devices Market Report
- Global Endometrial Ablation Devices market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Endometrial Ablation Devices trade, costs, and supply chains
- Endometrial Ablation Devices market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Endometrial Ablation Devices market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Endometrial Ablation Devices market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Endometrial Ablation Devices supply chain analysis
- Endometrial Ablation Devices trade analysis, Endometrial Ablation Devices market price analysis, and Endometrial Ablation Devices supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Endometrial Ablation Devices market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Johnson and Johnson Private Limited
- Medtronic PLC
- Stryker Corporation
- Boston Scientific Corporation
- Olympus Corporation
- Smith And Nephew PLC
- Hologic Inc.
- C R.Bard Inc.
- Cooper Companies Inc.
- Cook Medical
- Karl Storz GmbH And Co. KG
- Biotronik SE And Co. KG
- B. Braun Medical Inc.
- Richard Wolf GmbH
- Aesculap Inc.
- AngioDynamics
- Gyrus ACMI Inc.
- Minerva Surgical Inc.
- Minerva Surgical Inc.
- Lina Medical ApS
- Biolitec AG
- AEGEA Medical Inc.
- Albyn Medical
- Channel Medsystems Inc.
- CathRX Ltd.
- Ecleris Srl.
- Idoman Teoranta
- Surkon Medical Co. Ltd.
- Omnitech Systems Inc.
- Veldana Medical SA.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
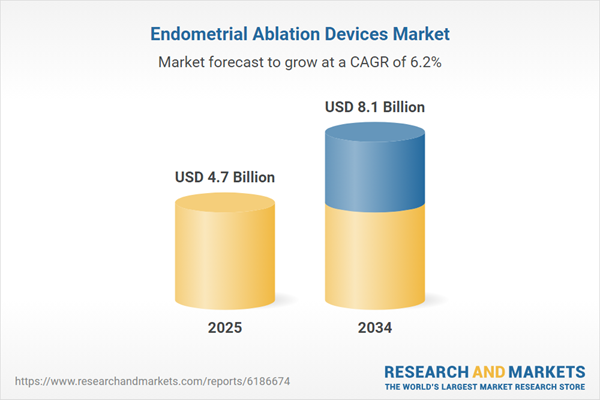
| Estimated Market Value ( USD | $ 4.7 Billion |
| Forecasted Market Value ( USD | $ 8.1 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |