The Thrombectomy Devices Market plays a vital role in the treatment of blood clots that can lead to life-threatening conditions such as stroke, pulmonary embolism, and deep vein thrombosis. Thrombectomy devices are medical tools designed to mechanically remove thrombi from blood vessels, restoring normal blood flow and preventing tissue damage. This market includes aspiration catheters, mechanical thrombectomy devices, ultrasonic devices, and combination systems used in both neurovascular and peripheral vascular applications. The growing global prevalence of cardiovascular diseases, particularly ischemic stroke, has placed thrombectomy as a frontline therapy for timely intervention. Demand for minimally invasive, rapid-recovery solutions is pushing hospitals and surgical centers to invest in cutting-edge thrombectomy systems, especially as clinical evidence supports their effectiveness in improving patient outcomes when performed within critical therapeutic windows.
The thrombectomy devices market experienced heightened activity due to continued innovation and favorable clinical trial results validating the use of mechanical thrombectomy in broader stroke patient populations. Expanded treatment guidelines by neurological associations encouraged the adoption of thrombectomy beyond the traditional six-hour window in select cases. Manufacturers launched next-generation devices with enhanced clot retrieval capabilities, improved navigation through tortuous vessels, and lower risk of vessel trauma. Hospitals integrated AI-based imaging solutions to rapidly identify eligible patients, further streamlining the triage and intervention process. Reimbursement policies were updated in several countries to support device-based treatment for acute ischemic strokes, which bolstered usage in both urban and secondary care settings. Additionally, academic collaborations accelerated R&D efforts around retrievable stents, aspiration-plus-mechanical systems, and drug-coated devices to increase clot removal efficiency while reducing procedural complications.
The thrombectomy devices market is poised for continued growth fueled by aging populations, expanded stroke care networks, and rising awareness among physicians about mechanical intervention efficacy. Innovations will focus on developing ultra-low-profile devices suitable for distal vessels, as well as hybrid solutions integrating drug delivery with mechanical clot extraction. AI-driven decision support tools will aid in rapid diagnosis, real-time navigation, and outcome prediction. The global expansion of neurovascular and interventional radiology capabilities will bring thrombectomy access to underserved regions, particularly in Asia-Pacific and Latin America. However, disparities in device affordability, operator training, and post-operative care infrastructure may limit equitable access and uniform clinical outcomes. Ensuring streamlined supply chains and cross-border regulatory alignment will be essential for maximizing the market’s impact on stroke and vascular disease management worldwide.
Key Insights: Thrombectomy Devices Market
- Clinical adoption of thrombectomy in extended time windows is increasing due to growing evidence of favorable outcomes in select stroke patients with salvageable brain tissue.
- Next-gen devices featuring improved deliverability and clot capture efficacy are gaining traction in complex vascular anatomy and large vessel occlusions.
- Integration of artificial intelligence in stroke imaging is supporting faster diagnosis and more accurate candidate selection for thrombectomy procedures.
- Combination therapies - pairing mechanical thrombectomy with thrombolytics or drug-coated tools - are being explored to enhance procedural effectiveness and reduce recurrence.
- Hospitals are establishing dedicated stroke response teams and regionalized stroke networks to increase patient access to thrombectomy-capable centers within therapeutic windows.
- Rising global incidence of ischemic strokes and cardiovascular events is driving urgent demand for effective, minimally invasive clot removal technologies.
- Expanding clinical trial data and updated treatment guidelines are supporting broader acceptance and utilization of thrombectomy in both acute and subacute settings.
- Growing investment in neurointerventional and vascular surgery infrastructure is boosting device procurement and training programs, especially in emerging economies.
- Supportive reimbursement frameworks and government stroke awareness campaigns are encouraging timely presentation and intervention for at-risk patients.
- The primary challenge facing the thrombectomy devices market is the lack of skilled interventionalists and consistent access to stroke-ready facilities in rural or resource-limited regions, which restricts widespread adoption and limits the potential to save lives through timely mechanical intervention.
Thrombectomy Devices Market Segmentation
By Product Type
- Aspiration Thrombectomy Devices
- Mechanical Thrombectomy Devices
- Ultrasonic Thrombectomy Devices
- Other Product Types
By Application
- Cardiovascular Application
- Peripheral Application
- Neurovascular Application
By End-User
- Hospitals
- Surgical centers
- Ambulatory
- Academia
- Contract Research Organizations (CROs)
Key Companies Analysed
- Medtronic plc
- Stryker Corporation
- Penumbra, Inc.
- Boston Scientific Corporation
- Terumo Corporation
- Teleflex Incorporated
- Johnson & Johnson (CERENOVUS)
- Inari Medical, Inc.
- AngioDynamics, Inc.
- MicroPort Scientific Corporation
Thrombectomy Devices Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.
Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.Thrombectomy Devices Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.
Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.Countries Covered
- North America - Thrombectomy Devices market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Thrombectomy Devices market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Thrombectomy Devices market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Thrombectomy Devices market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Thrombectomy Devices market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Thrombectomy Devices value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Thrombectomy Devices industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Thrombectomy Devices Market Report
- Global Thrombectomy Devices market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Thrombectomy Devices trade, costs, and supply chains
- Thrombectomy Devices market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Thrombectomy Devices market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Thrombectomy Devices market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Thrombectomy Devices supply chain analysis
- Thrombectomy Devices trade analysis, Thrombectomy Devices market price analysis, and Thrombectomy Devices supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Thrombectomy Devices market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Medtronic PLC
- Stryker Corporation
- Penumbra Inc.
- Boston Scientific Corporation
- Terumo Corporation
- Teleflex Incorporated
- Johnson & Johnson (CERENOVUS)
- Inari Medical Inc.
- AngioDynamics Inc.
- MicroPort Scientific Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
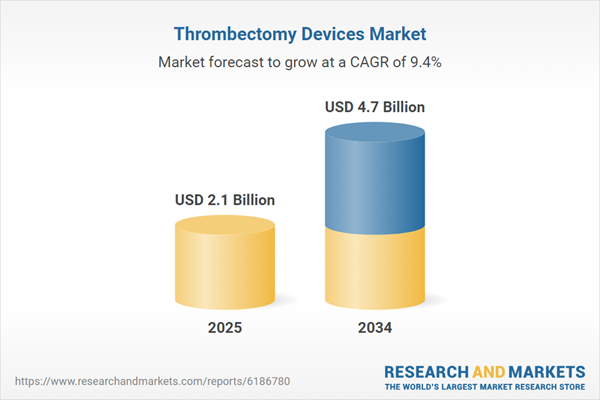
| Estimated Market Value ( USD | $ 2.1 Billion |
| Forecasted Market Value ( USD | $ 4.7 Billion |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |