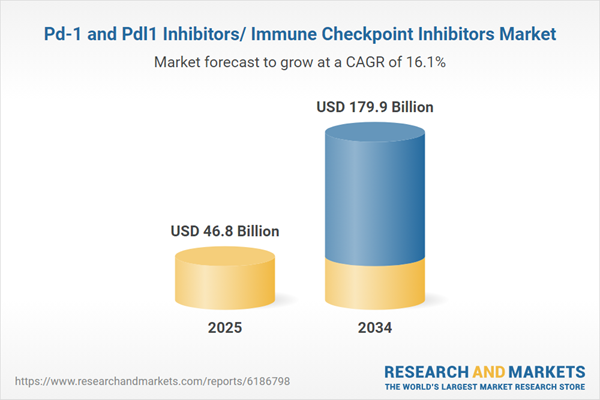
The global PD-1 and PD-L1 inhibitors market, also known as the immune checkpoint inhibitors market, is experiencing significant growth due to the rising prevalence of cancer and the increasing adoption of immunotherapy treatments. PD-1 (programmed death-1) and PD-L1 (programmed death-ligand 1) inhibitors are monoclonal antibodies that enhance the body’s immune response against tumors by blocking immune suppression pathways. These therapies have revolutionized cancer treatment, improving survival rates for patients with melanoma, lung cancer, bladder cancer, and other malignancies. Leading pharmaceutical companies such as Merck & Co., Bristol-Myers Squibb, Roche, and AstraZeneca are continuously investing in clinical trials to expand indications and improve combination therapy efficacy. The growing approval of PD-1/PD-L1 inhibitors for different tumor types and increasing patient access to immunotherapy are key drivers fueling market expansion. Additionally, biomarker-based precision medicine approaches are improving patient selection for immune checkpoint inhibitors, further strengthening their role in oncology treatment landscapes.
The PD-1 and PD-L1 inhibitors market has witnessed significant advancements, particularly in combination therapies, expanded indications, and next-generation checkpoint inhibitors. The FDA and EMA have approved new indications for PD-1/PD-L1 inhibitors, extending their use to additional tumor types such as gastric, head and neck, and triple-negative breast cancer. Combination therapy strategies, including PD-1 inhibitors with chemotherapy, targeted therapies, and novel immune-modulating agents, have gained traction, improving response rates and overcoming resistance mechanisms. The development of next-generation immune checkpoint inhibitors targeting multiple immune pathways, such as LAG-3 and TIGIT, has further enhanced the potential of immunotherapy in cancer treatment. Meanwhile, advances in companion diagnostics and predictive biomarkers have enabled better patient stratification, ensuring that PD-1/PD-L1 therapies are administered to patients most likely to benefit. Despite these innovations, challenges such as high treatment costs, immune-related adverse effects, and varying response rates among patients remain key concerns.
The PD-1 and PD-L1 inhibitors market is expected to evolve with the emergence of personalized immunotherapy, bispecific checkpoint inhibitors, and novel combinatorial approaches. AI-driven drug discovery will accelerate the development of next-generation checkpoint inhibitors, identifying new targets and optimizing therapeutic strategies. Bispecific immune checkpoint inhibitors, which target multiple immune regulatory pathways simultaneously, are expected to enhance tumor response and reduce resistance. The increasing adoption of liquid biopsy and circulating tumor DNA (ctDNA) analysis will improve early detection, real-time monitoring, and treatment personalization for immunotherapy patients. Additionally, regulatory bodies are likely to push for cost-effective biosimilars and novel pricing models to improve patient affordability and accessibility. As immuno-oncology continues to reshape cancer treatment, PD-1 and PD-L1 inhibitors will remain a cornerstone therapy, evolving alongside precision medicine to provide more effective and targeted cancer treatment solutions.
Key Insights: Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors Market
- Rise of Combination Immunotherapy Approaches: PD-1/PD-L1 inhibitors are increasingly combined with chemotherapy, targeted therapies, and immune-modulating agents to enhance treatment efficacy.
- Advancements in Next-Generation Checkpoint Inhibitors: New immune checkpoint targets, such as LAG-3 and TIGIT, are being explored to overcome resistance and improve patient response.
- Growth of Companion Diagnostics & Biomarker-Based Therapy Selection: Biomarker-driven patient stratification is improving treatment outcomes and reducing unnecessary exposure to immunotherapy.
- Expansion of PD-1/PD-L1 Inhibitor Indications Across Cancer Types: Regulatory approvals are increasing for additional malignancies, broadening the application of immune checkpoint inhibitors.
- Development of AI-Driven Drug Discovery for Immuno-Oncology: AI-powered research is accelerating the identification of new immune checkpoint targets and optimizing therapeutic combinations.
- Rising Cancer Incidence & Demand for Novel Therapies: The increasing global cancer burden is driving demand for effective immunotherapies such as PD-1 and PD-L1 inhibitors.
- Improved Understanding of Tumor Immunology & Resistance Mechanisms: Ongoing research into immune evasion mechanisms is shaping the development of more effective immune checkpoint therapies.
- Regulatory Approvals & Expanding Patient Access to Immunotherapy: Growing approvals for new indications and reimbursement policies are increasing accessibility to PD-1/PD-L1 therapies worldwide.
- Advancements in Precision Medicine & Biomarker-Guided Treatment: The use of predictive biomarkers is optimizing patient selection and improving immunotherapy success rates.
- High Treatment Costs & Affordability Concerns: The expensive nature of PD-1/PD-L1 inhibitors and combination therapies limits access for patients, necessitating pricing reforms and biosimilar development.
Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors Market Segmentation
By Product
- Nivolumab
- Pembrolizumab
- Atezolizumab
- Avelumab
- Durvalumab
By Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
By Application
- Lung Cancer
- Bladder Cancer
- Melanoma
- Hodgkin Lymphoma
- Colorectal Cancer
- Other Applications
By End-Users
- Hospitals
- Specialty Clinics
- Academic and Research Institutions
Key Companies Analysed
- Merck & Co., Inc. (Keytruda)
- Bristol Myers Squibb (Opdivo)
- Roche Holding AG (Tecentriq)
- Novartis AG
- AstraZeneca plc (Imfinzi)
- Pfizer Inc.
- Sanofi S.A.
- BeiGene Ltd.
- Incyte Corporation
- Eli Lilly and Company
Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors Market Report
- Global Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors trade, costs, and supply chains
- Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors supply chain analysis
- Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors trade analysis, Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market price analysis, and Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Pd-1 and Pdl1 Inhibitors/ Immune Checkpoint Inhibitors market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Merck & Co.
- Inc. (Keytruda)
- Bristol Myers Squibb (Opdivo)
- Roche Holding AG (Tecentriq)
- Novartis AG
- AstraZeneca PLC (Imfinzi)
- Pfizer Inc.
- Sanofi S.A.
- BeiGene Ltd.
- Incyte Corporation
- Eli Lilly and Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 46.8 Billion |
| Forecasted Market Value ( USD | $ 179.9 Billion |
| Compound Annual Growth Rate | 16.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |