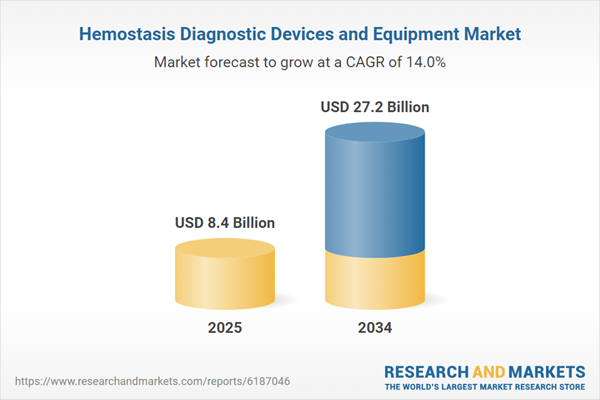
The Hemostasis Diagnostic Devices and Equipment Market is witnessing a significant expansion, driven by the growing global burden of bleeding disorders, cardiovascular conditions, and surgical interventions that necessitate accurate blood clotting analysis. These devices play a crucial role in evaluating the hemostatic function of patients, guiding treatment in critical care, emergency, and pre-operative settings. Innovations in coagulation analyzers, point-of-care testing kits, and automated laboratory systems are transforming the landscape of hemostasis diagnostics, ensuring faster and more precise results. As healthcare systems prioritize early detection and treatment of coagulation disorders such as hemophilia and thrombosis, demand for advanced diagnostic tools is on the rise. In both developed and developing markets, a growing focus on improving patient outcomes, coupled with increasing investments in healthcare infrastructure, continues to bolster the market's momentum. Rising awareness among clinicians and patients about the significance of timely diagnosis further supports this growth trajectory.
The Hemostasis Diagnostic Devices and Equipment Market experienced notable advancements in terms of product innovation and clinical application. The integration of AI and machine learning algorithms in diagnostic platforms improved test interpretation accuracy and workflow efficiency. Key industry players launched compact, user-friendly coagulation analyzers tailored for decentralized testing environments, enabling faster decision-making in critical care settings. Moreover, strategic collaborations between diagnostic companies and hospitals have led to enhanced product deployment and service accessibility, especially in emerging markets. Regulatory approvals of next-generation diagnostic kits and systems expanded the range of tests available for identifying rare bleeding disorders and monitoring anticoagulant therapy. Additionally, market players focused on developing devices that offer a combination of traditional laboratory capabilities with point-of-care functionality, providing flexibility across various clinical scenarios. These developments reflect the industry's efforts to address the evolving demands of modern healthcare systems and to improve diagnostic outcomes through technology-driven solutions.
The Hemostasis Diagnostic Devices and Equipment Market is expected to evolve further with a continued emphasis on automation, connectivity, and personalized medicine. Integration with digital health platforms and cloud-based data systems will facilitate real-time monitoring and remote diagnostics, enhancing patient care pathways. Advanced biosensors and miniaturized platforms are projected to gain traction, offering cost-effective and portable diagnostic options suitable for outpatient settings and resource-limited regions. Moreover, increasing research into biomarker discovery and multiplex testing will pave the way for comprehensive coagulation profiling and tailored therapies. Market growth will also be fueled by the aging global population, the rising prevalence of chronic conditions such as diabetes and cancer, and the expansion of universal healthcare programs in low- and middle-income countries. As competition intensifies, innovation will remain central to market differentiation, with companies investing in R&D to deliver faster, more accurate, and user-centric diagnostic solutions that align with the needs of modern healthcare ecosystems.
Key Insights: Hemostasis Diagnostic Devices and Equipment Market
- Adoption of point-of-care hemostasis testing is increasing across emergency and outpatient care settings due to its ability to deliver rapid and accurate results at the bedside.
- Artificial intelligence is being incorporated into hemostasis diagnostic platforms to assist clinicians in interpreting complex test results and predicting patient outcomes more efficiently.
- Manufacturers are focusing on developing compact and portable coagulation analyzers to meet the growing demand in resource-constrained and rural healthcare environments.
- Integration of diagnostic devices with electronic health records (EHRs) is enabling seamless data sharing, enhancing clinical decision-making and care coordination.
- There is a growing trend toward personalized diagnostics, with customized panels and biomarker-based testing gaining popularity for targeted therapy planning in coagulation disorders.
- Increasing prevalence of chronic diseases and conditions such as hemophilia, cancer, and cardiovascular disorders is propelling the demand for advanced hemostasis diagnostic tools.
- Rising surgical volumes and the need for preoperative coagulation screening are boosting the adoption of reliable and efficient diagnostic equipment in hospitals and clinics.
- Technological advancements in coagulation analyzers, including automation, connectivity, and AI integration, are enhancing the accuracy and efficiency of diagnostic processes.
- Expanding healthcare infrastructure and diagnostic capabilities in emerging economies are opening new avenues for market penetration and device deployment.
- High costs associated with advanced hemostasis diagnostic equipment and limited reimbursement in several regions continue to hinder widespread adoption, especially in low-income settings.
Hemostasis Diagnostic Devices and Equipment Market Segmentation
By Product
- Analyzers
- Coagulation Instrument
- Other Hemostasis Instruments
- Reagents and Kits
By Device Technology
- Automated
- Semi-Automated
- Manual
By End User
- Hospitals
- Clinics
- Independent Diagnostic
- Laboratories
- Other End Users
Key Companies Analysed
- C. R. Bard Inc.
- Baxter International Inc.
- Pfizer Inc.
- Johnson & Johnson
- Abbott Laboratories
- Beckman Coulter Inc.
- Becton Dickinson and Company
- Chrono-Log Corporation
- CSL Behring LLC
- F. Hoffmann-La Roche Ltd.
- Grifols S.A.
- HemCon Medical Technologies Inc.
- Integra Life Sciences Corporation
- Medtronic plc
- Siemens AG
- Thermo Fisher Scientific Inc.
- Trinity Biotech plc.
- Ethicon Inc.
- Boston Scientific Corporation
- Olympus Corporation
- Cook Group Inc.
- Halyard Health
- Medline Industries Inc.
- ConMed Corporation
- Moog Inc.
- Argon Medical Devices Inc.
- B. Braun Interventional Systems Inc.
- Bio-Rad Laboratories Inc.
- CareFusion Corporation
- Danaher Corporation
- DemeTECH Corporation
- Diagnostica Stago SAS
- Dolphin Sutures
- EndoEvolution LLC
- Haemonetics Corporation
- Internacional Farmacéutica S.A. de C.V.
- Merit Medical Systems Inc.
- Quidelortho Corp
- STAGO
- Stryker Corporation
Hemostasis Diagnostic Devices and Equipment Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Hemostasis Diagnostic Devices and Equipment Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Hemostasis Diagnostic Devices and Equipment market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Hemostasis Diagnostic Devices and Equipment market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Hemostasis Diagnostic Devices and Equipment market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Hemostasis Diagnostic Devices and Equipment market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Hemostasis Diagnostic Devices and Equipment market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Hemostasis Diagnostic Devices and Equipment value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Hemostasis Diagnostic Devices and Equipment industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Hemostasis Diagnostic Devices and Equipment Market Report
- Global Hemostasis Diagnostic Devices and Equipment market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Hemostasis Diagnostic Devices and Equipment trade, costs, and supply chains
- Hemostasis Diagnostic Devices and Equipment market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Hemostasis Diagnostic Devices and Equipment market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Hemostasis Diagnostic Devices and Equipment market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Hemostasis Diagnostic Devices and Equipment supply chain analysis
- Hemostasis Diagnostic Devices and Equipment trade analysis, Hemostasis Diagnostic Devices and Equipment market price analysis, and Hemostasis Diagnostic Devices and Equipment supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Hemostasis Diagnostic Devices and Equipment market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- C. R. Bard Inc.
- Baxter International Inc.
- Pfizer Inc.
- Johnson & Johnson
- Abbott Laboratories
- Beckman Coulter Inc.
- Becton Dickinson and Company
- Chrono-Log Corporation
- CSL Behring LLC
- F. Hoffmann-La Roche Ltd.
- Grifols S.A.
- HemCon Medical Technologies Inc.
- Integra Life Sciences Corporation
- Medtronic PLC
- Siemens AG
- Thermo Fisher Scientific Inc.
- Trinity Biotech PLC
- Ethicon Inc.
- Boston Scientific Corporation
- Olympus Corporation
- Cook Group Inc.
- Halyard Health
- Medline Industries Inc.
- ConMed Corporation
- Moog Inc.
- Argon Medical Devices Inc.
- B. Braun Interventional Systems Inc.
- Bio-Rad Laboratories Inc.
- CareFusion Corporation
- Danaher Corporation
- DemeTECH Corporation
- Diagnostica Stago SAS
- Dolphin Sutures
- EndoEvolution LLC
- Haemonetics Corporation
- Internacional Farmacéutica S.A. de C.V.
- Merit Medical Systems Inc.
- Quidelortho Corp
- STAGO
- Stryker Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 8.4 Billion |
| Forecasted Market Value ( USD | $ 27.2 Billion |
| Compound Annual Growth Rate | 13.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 40 |