The COVID-19 drug-associated APIs market rapidly emerged during the pandemic as pharmaceutical companies and researchers developed a range of treatments to address the virus. Active Pharmaceutical Ingredients (APIs) used in antiviral drugs, immune modulators, and supportive care medications formed the backbone of this market. With unprecedented global demand, the production of APIs increased, and manufacturers focused on scaling up their capacity, enhancing supply chain resilience, and ensuring high-quality standards. The market’s growth was also supported by government funding, public-private collaborations, and expedited regulatory approvals that allowed rapid access to these life-saving treatments.
The landscape of this market evolved significantly. While the initial urgency waned, the need for robust and scalable API manufacturing remained. Companies started to diversify their production portfolios, preparing for future pandemics and other public health emergencies. The emphasis shifted toward more cost-effective and sustainable production methods, including the use of green chemistry and continuous manufacturing technologies. Additionally, API manufacturers continued to explore partnerships with global suppliers to strengthen the reliability and stability of their supply chains, particularly in regions heavily affected by raw material shortages.
Looking ahead, the COVID-19 drug-associated APIs market is expected to transform further as the industry moves beyond pandemic-centric operations. The development of next-generation antiviral drugs, ongoing surveillance for new variants, and investments in long-term pandemic preparedness will keep demand stable. Furthermore, advancements in API manufacturing technologies, such as process intensification and synthetic biology, are likely to enhance efficiency, reduce costs, and improve environmental sustainability. These changes will position the API market as a critical component of the pharmaceutical supply chain, not only for COVID-19 treatments but for a broad spectrum of antiviral and immunomodulatory therapies.
Key Insights: COVID-19 Drug Associated Apis Market
- Adoption of green chemistry and sustainable manufacturing practices.
- Increased use of continuous manufacturing technologies to improve efficiency.
- Strengthening supply chain resilience through global partnerships and diversification.
- Development of APIs for next-generation antiviral drugs.
- Expansion of API production capacity to address future pandemics.
- Ongoing demand for antiviral and immune modulating APIs due to variant emergence.
- Government funding and incentives to enhance domestic API manufacturing.
- Technological advancements improving cost efficiency and scalability.
- Global collaborations ensuring a steady supply of high-quality raw materials.
- Managing production costs in the face of fluctuating raw material prices.
- Meeting stringent regulatory and quality standards for new API formulations.
- Addressing environmental concerns associated with traditional API production methods.
COVID-19 Drug Associated Apis Market Segmentation
By Drug Class
- Antimalarials
- Bronchodilators
- Antibiotics
- Antivirals
- Other Drug Class
By Synthesis Type
- Synthetic
- Biotech
By Business Mode
- Captive API
- Merchant API
Key Companies Analysed
- Dr. Reddy's Laboratories Ltd.
- Lianyungang Guike Pharmaceutical CO. LTD.
- Alembic Pharmaceuticals Ltd.
- Wockhardt Ltd.
- Sandoz Srl
- Lupin Limited
- Aurobindo Pharma Ltd.
- Shanghai Shyndec Pharmaceutical Co. Ltd.
- Zhejiang Yatai Pharmaceutical Co. Ltd.
- Zhejiang Guobang Pharmaceutical Co Ltd.
- Cipla Limited
- Shandong Boyuan Pharmaceutical Co. Ltd.
- Aspiro Pharma Ltd.
- Qilu Antibiotics Pharmaceutical Co. Ltd.
- Shenzhen China Resources Jiuxin Pharmaceutical Co. Ltd.
- Zhejiang Cheng Yi Pharmaceutical
- Star Lake Bioscience Co.Inc.
- Hetero Labs Ltd.
- Geno Pharmaceuticals Ltd.
- Mylan Laboratories Ltd.
- Ipca Laboratories Limited
- Zydus Takeda Healthcare Private Limited
- Mangalam Drugs & Organics
- Wallace Pharmaceuticals
- Zhejiang Hisun Pharmaceutical Co. Ltd.
- Zhejiang Huahai Pharmaceutical Co. Ltd.
- Zhejiang Jiuzhou Pharmaceutical Co. Ltd.
- Zhejiang NHU Co. Ltd.
- Zhejiang Xianju Pharmaceutical Co. Ltd.
- Zhejiang Yongtai Technology Co. Ltd.
- Zhejiang Zhebei Pharmaceutical Co. Ltd.
- Zhejiang Tianyu Pharmaceutical Co. Ltd.
- Zhejiang Aisheng Pharmaceutical Co. Ltd.
- Zhejiang Aokang Pharmaceutical Co. Ltd.
- Zhejiang Anji Huifeng Surgical Dressings Co. Ltd.
COVID-19 Drug Associated Apis Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
COVID-19 Drug Associated Apis Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - COVID-19 Drug Associated Apis market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - COVID-19 Drug Associated Apis market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - COVID-19 Drug Associated Apis market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - COVID-19 Drug Associated Apis market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - COVID-19 Drug Associated Apis market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the COVID-19 Drug Associated Apis value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the COVID-19 Drug Associated Apis industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the COVID-19 Drug Associated Apis Market Report
- Global COVID-19 Drug Associated Apis market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on COVID-19 Drug Associated Apis trade, costs, and supply chains
- COVID-19 Drug Associated Apis market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- COVID-19 Drug Associated Apis market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term COVID-19 Drug Associated Apis market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and COVID-19 Drug Associated Apis supply chain analysis
- COVID-19 Drug Associated Apis trade analysis, COVID-19 Drug Associated Apis market price analysis, and COVID-19 Drug Associated Apis supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest COVID-19 Drug Associated Apis market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Dr. Reddy's Laboratories Ltd.
- Lianyungang Guike Pharmaceutical CO. Ltd.
- Alembic Pharmaceuticals Ltd.
- Wockhardt Ltd.
- Sandoz Srl
- Lupin Limited
- Aurobindo Pharma Ltd.
- Shanghai Shyndec Pharmaceutical Co. Ltd.
- Zhejiang Yatai Pharmaceutical Co. Ltd.
- Zhejiang Guobang Pharmaceutical Co Ltd.
- Cipla Limited
- Shandong Boyuan Pharmaceutical Co. Ltd.
- Aspiro Pharma Ltd.
- Qilu Antibiotics Pharmaceutical Co. Ltd.
- Shenzhen China Resources Jiuxin Pharmaceutical Co. Ltd.
- Zhejiang Cheng Yi Pharmaceutical
- Star Lake Bioscience Co.Inc.
- Hetero Labs Ltd.
- Geno Pharmaceuticals Ltd.
- Mylan Laboratories Ltd.
- Ipca Laboratories Limited
- Zydus Takeda Healthcare Private Limited
- Mangalam Drugs & Organics
- Wallace Pharmaceuticals
- Zhejiang Hisun Pharmaceutical Co. Ltd.
- Zhejiang Huahai Pharmaceutical Co. Ltd.
- Zhejiang Jiuzhou Pharmaceutical Co. Ltd.
- Zhejiang NHU Co. Ltd.
- Zhejiang Xianju Pharmaceutical Co. Ltd.
- Zhejiang Yongtai Technology Co. Ltd.
- Zhejiang Zhebei Pharmaceutical Co. Ltd.
- Zhejiang Tianyu Pharmaceutical Co. Ltd.
- Zhejiang Aisheng Pharmaceutical Co. Ltd.
- Zhejiang Aokang Pharmaceutical Co. Ltd.
- Zhejiang Anji Huifeng Surgical Dressings Co. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
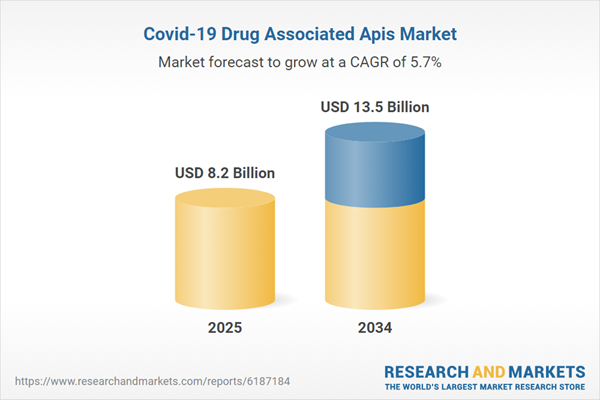
| Estimated Market Value ( USD | $ 8.2 Billion |
| Forecasted Market Value ( USD | $ 13.5 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 35 |