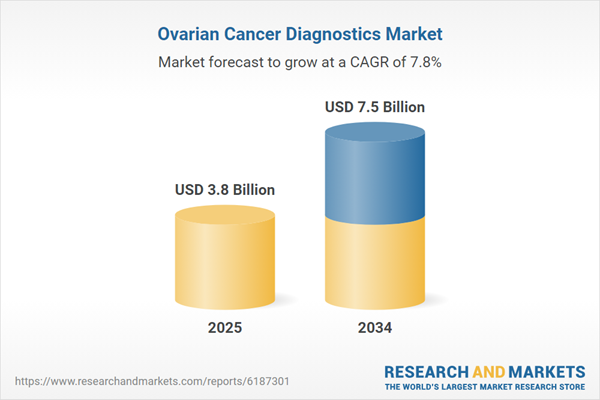
The ovarian cancer diagnostics market is experiencing steady growth, driven by increasing awareness, technological advancements, and rising incidences of ovarian cancer worldwide. Ovarian cancer is often diagnosed in its later stages due to the lack of early symptoms, making early and accurate detection crucial for improving patient outcomes. The demand for advanced diagnostic solutions is rising as healthcare providers seek non-invasive, cost-effective, and highly sensitive testing methods. Traditional diagnostic techniques, such as transvaginal ultrasound and CA-125 blood tests, are being complemented by newer approaches, including liquid biopsy, genetic testing, and artificial intelligence (AI)-assisted imaging analysis. Governments and healthcare organizations are also investing in screening programs and research initiatives to improve early detection rates. With the integration of AI, molecular diagnostics, and biomarker-based screening tools, the ovarian cancer diagnostics market is poised for continuous expansion in the coming years.
The ovarian cancer diagnostics market saw significant advancements in precision medicine, AI-driven diagnostics, and biomarker research. The use of liquid biopsy gained traction, offering a minimally invasive method for detecting circulating tumor DNA (ctDNA) and other cancer-related biomarkers. AI-powered imaging analysis improved the accuracy of ultrasound and MRI scans, enabling radiologists to detect malignancies with higher precision. Advances in next-generation sequencing (NGS) allowed for more comprehensive genetic profiling of ovarian cancer, helping identify patients who may benefit from targeted therapies. The year also saw increased collaboration between diagnostic companies and pharmaceutical firms to develop companion diagnostics, ensuring more personalized treatment approaches. Governments and private organizations launched new awareness campaigns, emphasizing the importance of early screening in high-risk populations. Additionally, regulatory agencies fast-tracked approvals for innovative diagnostic tools, improving accessibility to cutting-edge testing methods.
The ovarian cancer diagnostics market is expected to see further growth in AI-assisted diagnostic tools, personalized screening methods, and point-of-care testing innovations. The expansion of multi-omics approaches, including proteomics and metabolomics, will enhance the accuracy of early detection strategies. Wearable health technology is also anticipated to play a role in continuous monitoring and early warning systems for ovarian cancer risk. Researchers are exploring the potential of novel biomarkers that could provide even earlier detection, reducing the mortality rate associated with late-stage diagnoses. The integration of cloud-based diagnostics and telemedicine will improve access to ovarian cancer screening, particularly in remote and underserved areas. As genetic screening becomes more affordable, more individuals will opt for proactive testing to assess their hereditary risk factors. With continued advancements in biotechnology and artificial intelligence, the ovarian cancer diagnostics market is set to evolve, offering more efficient and accessible solutions for early detection and disease management.
Key Insights: Ovarian Cancer Diagnostics Market
- Rise of Liquid Biopsy for Early Detection: The adoption of liquid biopsy techniques is increasing due to their minimally invasive nature, allowing for early-stage ovarian cancer detection through circulating tumor DNA analysis.
- AI-Driven Imaging and Diagnostic Interpretation: Artificial intelligence is enhancing imaging-based diagnostics, improving the accuracy of ultrasound, MRI, and CT scans for better cancer detection.
- Expansion of Companion Diagnostics: More diagnostic tests are being developed in collaboration with pharmaceutical companies to ensure targeted therapies are tailored to specific genetic profiles.
- Multi-Omics Approach in Ovarian Cancer Screening: The integration of genomics, proteomics, and metabolomics is improving the accuracy of diagnostic methods, enabling more comprehensive cancer risk assessment.
- Growth of At-Home and Point-of-Care Testing: The development of user-friendly diagnostic kits is increasing accessibility to ovarian cancer screening, especially for high-risk individuals in remote areas.
- Rising Incidence of Ovarian Cancer Worldwide: The increasing prevalence of ovarian cancer is driving demand for improved diagnostic tools and early detection methods.
- Advancements in Molecular Diagnostics and Genetic Testing: Innovations in biomarker research and genetic screening are enhancing the precision and reliability of ovarian cancer detection.
- Growing Investment in Cancer Research and Awareness Campaigns: Government initiatives and non-profit organizations are supporting early screening programs and funding diagnostic advancements.
- Increasing Adoption of AI and Machine Learning in Healthcare: The use of AI-driven diagnostic solutions is improving the speed and accuracy of ovarian cancer detection, leading to better patient outcomes.
- High Cost of Advanced Diagnostic Technologies: The expense associated with cutting-edge diagnostic tools, including genetic testing and AI-driven imaging, can limit accessibility, particularly in low-income regions.
Ovarian Cancer Diagnostics Market Segmentation
By Product Type
- Instruments
- Kits
- Reagents
By Diagnosis Type
- Biopsy
- Blood Test
- Imaging
- Other Diagnosis Types
By Cancer Type
- Epithelial Tumor
- Germ Cell Tumor
- Stromal Cell Tumor
- Other Cancer Types
By End User
- Cancer Diagnostic Centers
- Hospital Laboratories
- Research Institutes
Key Companies Analysed
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- AstraZeneca plc
- Abbott Laboratories
- GlaxoSmithKline plc
- Eli Lilly and Company
- GE HealthCare Technologies Inc.
- Quest Diagnostics Incorporated
- Agilent Technologies Inc.
- Hologic Inc.
- Illumina Inc.
- PerkinElmer Inc.
- Sysmex Corporation
- Bio-rad Laboratories Inc.
- Qiagen N.V.
- Natera Inc.
- The American Cancer Society
- Myriad Genetics Inc.
- ArcherDX Inc.
- Invitae Corporation
- Guardant Health
- Luminex Corporation
- NanoString Technologies Inc.
- Siemens Healthcare Private Limited
- Menarini Silicon Biosystems S.p.A.
- Precipio Inc.
- Angle plc
Ovarian Cancer Diagnostics Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Ovarian Cancer Diagnostics Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Ovarian Cancer Diagnostics market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Ovarian Cancer Diagnostics market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Ovarian Cancer Diagnostics market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Ovarian Cancer Diagnostics market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Ovarian Cancer Diagnostics market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Ovarian Cancer Diagnostics value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Ovarian Cancer Diagnostics industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Ovarian Cancer Diagnostics Market Report
- Global Ovarian Cancer Diagnostics market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Ovarian Cancer Diagnostics trade, costs, and supply chains
- Ovarian Cancer Diagnostics market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Ovarian Cancer Diagnostics market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Ovarian Cancer Diagnostics market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Ovarian Cancer Diagnostics supply chain analysis
- Ovarian Cancer Diagnostics trade analysis, Ovarian Cancer Diagnostics market price analysis, and Ovarian Cancer Diagnostics supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Ovarian Cancer Diagnostics market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- AstraZeneca PLC
- Abbott Laboratories
- GlaxoSmithKline PLC
- Eli Lilly and Company
- GE HealthCare Technologies Inc.
- Quest Diagnostics Incorporated
- Agilent Technologies Inc.
- Hologic Inc.
- Illumina Inc.
- PerkinElmer Inc.
- Sysmex Corporation
- Bio-rad Laboratories Inc.
- Qiagen N.V.
- Natera Inc.
- The American Cancer Society
- Myriad Genetics Inc.
- ArcherDX Inc.
- Invitae Corporation
- Guardant Health
- Luminex Corporation
- NanoString Technologies Inc.
- Siemens Healthcare Private Limited
- Menarini Silicon Biosystems S.p.A.
- Precipio Inc.
- Angle PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 3.8 Billion |
| Forecasted Market Value ( USD | $ 7.5 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |