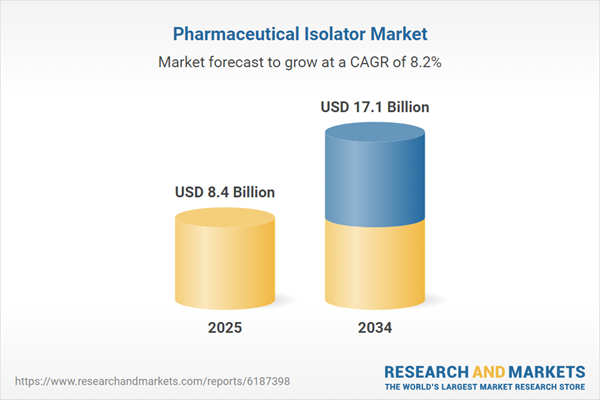
The pharmaceutical isolator market is growing rapidly due to the increasing need for contamination control in drug manufacturing and aseptic processing. Isolators are enclosed, sealed environments designed to protect pharmaceutical products from microbial contamination and ensure compliance with stringent regulatory requirements. These systems are used extensively in sterile drug manufacturing, compounding pharmacies, and laboratory research to enhance product quality and operator safety. The rising demand for high-potency active pharmaceutical ingredients (HPAPIs) and biologics has further fueled the adoption of pharmaceutical isolators, as these sensitive compounds require an ultra-clean and controlled manufacturing environment. North America and Europe dominate the market due to strong regulatory enforcement, advanced pharmaceutical manufacturing facilities, and high investment in automation technologies. Meanwhile, Asia-Pacific is witnessing increased adoption, driven by the expansion of pharmaceutical production and the growing implementation of Good Manufacturing Practices (GMP). As pharmaceutical companies strive to meet evolving industry standards, the demand for innovative, fully automated, and modular isolator systems is expected to rise.
The pharmaceutical isolator market experienced significant advancements in automation, real-time monitoring, and material innovations. The integration of robotic arms and AI-driven quality control systems within isolators enhanced precision in aseptic drug manufacturing, reducing human intervention and minimizing contamination risks. Single-use isolators gained popularity, especially in contract manufacturing organizations (CMOs) and small-scale production, offering greater flexibility and reduced cleaning validation requirements. Regulatory agencies such as the FDA and EMA reinforced stringent sterility assurance standards, prompting pharmaceutical companies to upgrade their containment systems to meet evolving compliance requirements. Additionally, sustainability became a key focus, with manufacturers exploring energy-efficient isolator designs and eco-friendly filtration systems to minimize environmental impact. The increased demand for cell and gene therapy production also drove the adoption of advanced isolator systems, ensuring a contamination-free environment for personalized medicine and regenerative therapies. Despite these advancements, supply chain disruptions and rising raw material costs presented challenges for isolator manufacturers, impacting production timelines and pricing.
The pharmaceutical isolator market is expected to witness further innovations in AI-driven monitoring, closed-system automation, and modular isolator designs. AI and machine learning will play a greater role in predictive maintenance, enabling pharmaceutical manufacturers to anticipate system failures and optimize operational efficiency. The adoption of real-time environmental monitoring and smart sensors will enhance sterility assurance, reducing risks associated with microbial contamination. The Asia-Pacific region is projected to see rapid growth, driven by increasing investments in pharmaceutical infrastructure and rising regulatory enforcement for aseptic drug production. The expansion of personalized medicine and highly sensitive biologics will necessitate the development of isolators with advanced containment capabilities to handle ultra-potent and highly reactive compounds. Sustainability initiatives will gain momentum, with the implementation of energy-efficient HVAC systems and recyclable materials in isolator design. However, the complexity of regulatory compliance and the high initial investment costs associated with pharmaceutical isolators will remain key challenges, requiring continued innovation and industry collaboration to ensure cost-effective and compliant solutions.
Key Insights: Pharmaceutical Isolator Market
- Integration of Robotics and AI-Driven Automation: The use of robotic arms and AI-powered quality control systems is enhancing sterility assurance, minimizing human intervention, and improving precision in aseptic drug manufacturing.
- Rising Adoption of Single-Use Isolators: The demand for flexible, disposable isolator systems is increasing, particularly in CMOs and personalized medicine production, due to their cost-effectiveness and ease of cleaning validation.
- Advancements in Real-Time Environmental Monitoring: Smart sensors and real-time data analytics are being integrated into isolators to enhance contamination control, ensuring consistent sterility in pharmaceutical manufacturing.
- Growth in Personalized Medicine and Biologics Manufacturing: The expansion of cell and gene therapies is driving the need for high-containment isolators to ensure sterility in ultra-sensitive drug production environments.
- Focus on Sustainability and Energy-Efficient Isolator Designs: Manufacturers are investing in eco-friendly filtration systems, reduced energy consumption, and recyclable isolator components to align with global sustainability goals.
- Stringent Regulatory Requirements for Sterile Manufacturing: Increasing enforcement of GMP and sterility assurance standards by agencies such as the FDA and EMA is driving the adoption of advanced isolator technologies.
- Growing Demand for High-Potency APIs and Biopharmaceuticals: The need for containment systems capable of handling potent and sensitive drug compounds is fueling investment in pharmaceutical isolators.
- Advancements in Automation and Process Optimization: The integration of automated control systems, smart monitoring, and AI-driven maintenance is enhancing efficiency and reducing operational risks in isolator technology.
- Expansion of Pharmaceutical Manufacturing in Emerging Markets: The rapid growth of the pharmaceutical industry in Asia-Pacific and Latin America is increasing demand for isolators to support sterile drug production facilities.
- High Initial Investment and Compliance Costs: The cost-intensive nature of isolator technology, along with the complexity of meeting evolving regulatory requirements, poses challenges for pharmaceutical manufacturers seeking to upgrade their containment systems.
Pharmaceutical Isolator Market Segmentation
By Type
- Closed Isolator Systems
- Open Isolator Systems
By Configuration
- Floor-Standing
- Modular
- Mobile
- Compact
- Tabletop
- Portable
By Application
- Aseptic Isolators
- Containment Isolators
- Other Applications
By End-User
- Pharmaceutical and Biotechnology Companies
- Research Laboratories
- Other End Users
Key Companies Analysed
- Getinge AB
- Wabash National Corporation
- Chiyoda Corporation
- Azbil Corporation
- Hosokawa micron Ltd.
- Bioquell Limited
- M Braun Inertgas-Systeme GmbH
- Fedegari Group
- Extract Technology Ltd.
- Germfree Laboratories Inc.
- COMECER SpA
- Hosokawa Micron Powder Systems
- ACIC Pharmaceutical Machinery
- Skan AG
- Custom Powder Systems
- NuAire Limited
- Gelman Singapore Pte Ltd.
- Contained Air Solutions Ltd.
- Dec Group
- ITECO Engineering Srl
- Schematic Engineering Industries Private Limited.
- Containment Technologies Group Inc.
- Inert Corporation
Pharmaceutical Isolator Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Pharmaceutical Isolator Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Pharmaceutical Isolator market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Pharmaceutical Isolator market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Pharmaceutical Isolator market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Pharmaceutical Isolator market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Pharmaceutical Isolator market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Pharmaceutical Isolator value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Pharmaceutical Isolator industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Pharmaceutical Isolator Market Report
- Global Pharmaceutical Isolator market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Pharmaceutical Isolator trade, costs, and supply chains
- Pharmaceutical Isolator market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Pharmaceutical Isolator market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Pharmaceutical Isolator market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Pharmaceutical Isolator supply chain analysis
- Pharmaceutical Isolator trade analysis, Pharmaceutical Isolator market price analysis, and Pharmaceutical Isolator supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Pharmaceutical Isolator market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Getinge AB
- Wabash National Corporation
- Chiyoda Corporation
- Azbil Corporation
- Hosokawa micron Ltd.
- Bioquell Limited
- M Braun Inertgas-Systeme GmbH
- Fedegari Group
- Extract Technology Ltd.
- Germfree Laboratories Inc.
- COMECER SpA
- Hosokawa Micron Powder Systems
- ACIC Pharmaceutical Machinery
- Skan AG
- Custom Powder Systems
- NuAire Limited
- Gelman Singapore Pte Ltd.
- Contained Air Solutions Ltd.
- Dec Group
- ITECO Engineering Srl
- Schematic Engineering Industries Private Limited.
- Containment Technologies Group Inc.
- Inert Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 8.4 Billion |
| Forecasted Market Value ( USD | $ 17.1 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |