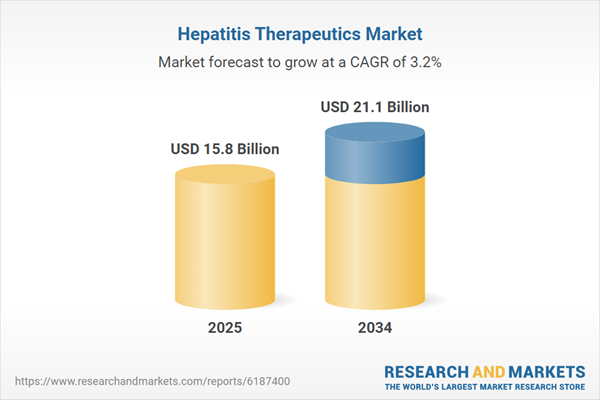
The Hepatitis Therapeutics Market continues to evolve as global health agencies, pharmaceutical companies, and governments intensify their focus on managing the burden of viral hepatitis, particularly types B and C. Increasing prevalence rates, especially in low- and middle-income countries, have driven demand for effective antiviral medications, vaccines, and public awareness campaigns. Therapeutic landscape includes a broad range of antiviral agents, immunomodulators, and interferons, with recent advancements favoring direct-acting antivirals (DAAs) that offer shorter treatment durations and higher cure rates. While Hepatitis C has witnessed substantial progress due to DAAs, unmet needs in Hepatitis B and other less common strains still offer room for innovation. Biopharma collaborations, enhanced screening programs, and supportive regulatory frameworks are helping to bridge gaps in diagnosis and treatment access, bolstering market growth globally.
The Hepatitis Therapeutics Market experienced notable momentum driven by wider adoption of novel oral therapies, integration of real-world evidence into treatment guidelines, and ongoing public health campaigns targeting early detection. Major players expanded patient access initiatives, particularly in developing nations where Hepatitis B and C infections remain endemic. The year also saw accelerated approvals of combination therapies offering pan-genotypic coverage, simplifying treatment regimens across various hepatitis virus strains. Digital health tools, including mobile-based adherence platforms, gained traction for supporting patient engagement. In addition, partnerships between public and private sectors led to increased funding for hepatitis elimination programs aligned with WHO’s 2030 goals. With greater emphasis on personalized medicine, 2024 positioned the market on a strong trajectory toward broader therapeutic reach and improved patient outcomes.
The Hepatitis Therapeutics Market is expected to undergo transformative growth driven by emerging technologies such as RNA interference and gene editing therapies, offering potential functional cures, particularly for chronic Hepatitis B. Biopharmaceutical innovation will likely introduce next-generation antivirals with improved resistance profiles and reduced side effects. Investment in decentralized testing and point-of-care diagnostics will play a vital role in reaching underserved populations, especially in rural regions. Strategic expansion into Asia-Pacific and African markets, where hepatitis prevalence is highest, will be crucial for global market penetration. Additionally, the integration of artificial intelligence into disease surveillance and treatment optimization will enhance clinical decision-making. As countries implement universal screening policies and scale up preventive vaccination, the long-term outlook for the hepatitis therapeutics market remains optimistic with significant opportunities for pharmaceutical advancements and public health impact.
Key Insights: Hepatitis Therapeutics Market
- Wider adoption of pan-genotypic direct-acting antivirals is reshaping treatment protocols by offering simplified, single-regimen solutions that are effective across all major hepatitis C genotypes.
- Public-private collaborations are intensifying, with governments partnering with pharma companies to expand access to hepatitis therapies in low-income and high-burden regions.
- Personalized medicine approaches are gaining ground, leveraging genomic data to tailor hepatitis treatment regimens for improved efficacy and reduced side effects.
- Decentralized testing and mobile diagnostics are becoming increasingly prevalent, making early detection and timely treatment initiation more accessible in remote areas.
- Advancements in long-acting injectables and therapeutic vaccines are emerging as promising alternatives to daily oral medications in hepatitis management.
- Rising global prevalence of hepatitis infections, particularly in Asia-Pacific and Sub-Saharan Africa, is creating sustained demand for effective therapeutic solutions.
- Government initiatives, including national hepatitis elimination plans and WHO-led campaigns, are catalyzing investments in treatment and prevention programs.
- Strong pipeline of novel therapies, including RNA-based treatments and immune modulators, is fueling innovation and expanding clinical trial activity in the market.
- Improved healthcare infrastructure and expanded insurance coverage in emerging markets are enhancing access to diagnostics and treatment for hepatitis patients.
- Limited awareness and late diagnosis remain key challenges, especially in rural and underserved regions, leading to delayed treatment and higher disease transmission rates.
Hepatitis Therapeutics Market Segmentation
By Disease Type
- Hepatitis A
- Hepatitis B
- Hepatitis C
- Other Disease Types
By Drug Class
- Oral Antivirals
- Immune Modulators
By Distribution Channel
- Hospital Pharmacies
- Drug Stores and Retail Pharmacies
- Online Providers
Key Companies Analysed
- Gilead Sciences Inc.
- Bristol-Myers Squibb Co.
- AbbVie Inc.
- Merck & Co. Inc.
- Johnson & Johnson Services Inc.
- Cipla Inc.
- F Hoffmann-La Roche Ltd.
- Biocon Limited
- LAURUS Labs Ltd.
- Zydus Lifesciences Limited
- Hetero Healthcare Limited
- Novartis AG
- Sanofi SA
- GlaxoSmithKline plc
- Lupin Ltd.
- Teva Pharmaceutical Industries Ltd.
- NATCO Pharma Limited
- Mylan NV
- Pfizer Inc.
- AstraZeneca plc
- Bayer AG
- Boehringer Ingelheim International GmbH
- Eli Lilly and Company
- Novo Nordisk A/S
- Takeda Pharmaceutical Company Limited
- Biogen Inc.
- Vertex Pharmaceuticals Incorporated
- Biocon Biopharmaceuticals Pvt. Ltd.
- Zealand Pharma A/S
- Zenyaku Kogyo Co. Ltd.
- Zosano Pharma Corporation.
Hepatitis Therapeutics Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Hepatitis Therapeutics Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Hepatitis Therapeutics market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Hepatitis Therapeutics market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Hepatitis Therapeutics market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Hepatitis Therapeutics market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Hepatitis Therapeutics market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Hepatitis Therapeutics value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Hepatitis Therapeutics industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Hepatitis Therapeutics Market Report
- Global Hepatitis Therapeutics market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Hepatitis Therapeutics trade, costs, and supply chains
- Hepatitis Therapeutics market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Hepatitis Therapeutics market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Hepatitis Therapeutics market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Hepatitis Therapeutics supply chain analysis
- Hepatitis Therapeutics trade analysis, Hepatitis Therapeutics market price analysis, and Hepatitis Therapeutics supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Hepatitis Therapeutics market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Gilead Sciences Inc.
- Bristol-Myers Squibb Co.
- AbbVie Inc.
- Merck & Co. Inc.
- Johnson & Johnson Services Inc.
- Cipla Inc.
- F Hoffmann-La Roche Ltd.
- Biocon Limited
- LAURUS Labs Ltd.
- Zydus Lifesciences Limited
- Hetero Healthcare Limited
- Novartis AG
- Sanofi SA
- GlaxoSmithKline PLC
- Lupin Ltd.
- Teva Pharmaceutical Industries Ltd.
- NATCO Pharma Limited
- Mylan NV
- Pfizer Inc.
- AstraZeneca PLC
- Bayer AG
- Boehringer Ingelheim International GmbH
- Eli Lilly and Company
- Novo Nordisk A/S
- Takeda Pharmaceutical Company Limited
- Biogen Inc.
- Vertex Pharmaceuticals Incorporated
- Biocon Biopharmaceuticals Pvt. Ltd.
- Zealand Pharma A/S
- Zenyaku Kogyo Co. Ltd.
- Zosano Pharma Corporation .
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 15.8 Billion |
| Forecasted Market Value ( USD | $ 21.1 Billion |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |