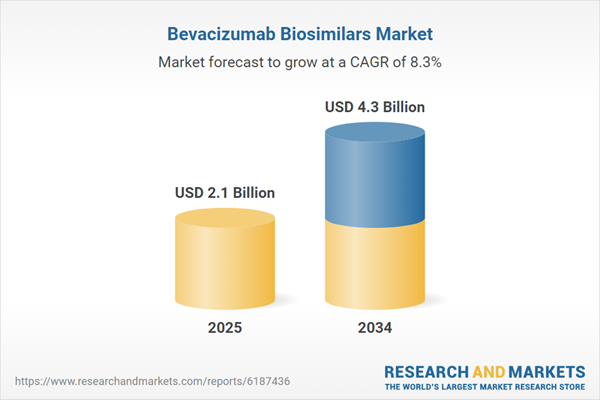
The bevacizumab biosimilars market is rapidly growing as healthcare systems worldwide seek cost-effective alternatives to branded biologics. Bevacizumab, an anti-vascular endothelial growth factor (anti-VEGF) monoclonal antibody, is widely used to treat various cancers, including colorectal, lung, and kidney cancers, as well as certain ophthalmic conditions. Biosimilars offer a more affordable option while maintaining comparable efficacy and safety profiles, making them an attractive choice for patients, providers, and payers.
In recent years, regulatory approvals and increasing confidence in biosimilar safety have driven market expansion. Several bevacizumab biosimilars have gained approval in key regions such as the U.S., Europe, and Asia, and their uptake is steadily increasing. These approvals have been supported by rigorous clinical studies demonstrating equivalence to the reference product in terms of efficacy, safety, and immunogenicity. As patents for the reference biologic expire, more biosimilar versions are entering the market, intensifying competition and driving down costs.
Regionally, Europe has led the adoption of bevacizumab biosimilars, supported by well-established regulatory pathways, favorable pricing policies, and broad physician acceptance. North America is catching up as payers and healthcare providers increasingly recognize the value of biosimilars in reducing treatment costs. Meanwhile, emerging markets in Asia-Pacific and Latin America are seeing rapid growth due to rising cancer prevalence and efforts to expand access to advanced therapies. With ongoing developments in manufacturing, regulation, and education, the bevacizumab biosimilars market is poised for sustained growth and increased accessibility.
Key Insights: Bevacizumab Biosimilars Market
- Increasing regulatory approvals of bevacizumab biosimilars in major markets.
- Growing physician and patient confidence in biosimilar safety and efficacy.
- Rising investment in biosimilar development and production by pharmaceutical companies.
- High cost of branded biologics, creating demand for more affordable alternatives.
- Expanding cancer prevalence and the need for broader access to anti-VEGF therapies.
- Regulatory frameworks encouraging biosimilar development and adoption.
- Intense competition among biosimilar manufacturers, driving price pressures.
- Resistance from some healthcare providers and patients due to misconceptions about biosimilar efficacy.
- Complexities in manufacturing and ensuring consistent quality, requiring significant investment in production capabilities.
Bevacizumab Biosimilars Market Segmentation
By Product
- Avastin
- Mvasi
- Zirabev
- Aybintio
- Other Products
By Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
- Other Distribution Channels
By Application
- Colorectal Cancer
- Non-small Cell Lung Cancer
- Glioblastoma
- Renal Cell Carcinoma
- Cervical Cancer
- Ovarian Cancer
Key Companies Analysed
- Cipla Limited
- Reliance lifesciences Pvt. Ltd.
- Genentech Inc
- Fujifilm Kyowa Kirin Biologics Co. Ltd.
- Pfizer Inc.
- Abbvie Inc.
- Amgen Inc.
- F. Hoffmann-La Roche Ltd.
- Teva Pharmaceutical Industries Ltd.
- Mylan Inc.
- Daiichi Sankyo Company Limited
- Beaconpharma Ltd.
- Innovent Biologics Inc.
- STADA Arzneimittel AG
- Shanghai Henlius Biotech Inc.
- Aurobindo Pharma Ltd.
- Dr. Reddy's Laboratories Ltd.
- Biocon Ltd.
- Intas Pharmaceuticals Ltd.
- Amneal Pharmaceuticals LLC
- Samsung Bioepis Co. Ltd.
- Celltrion Inc.
- Coherus BioSciences Inc.
- Hetero Drugs Ltd.
- EirGen Pharma Ltd.
- Allergan Plc.
- Apotex Inc.
- BioXpress Therapeutics SA
- Celgene Corporation Co. Ltd.
- mAbxience SA
Bevacizumab Biosimilars Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Bevacizumab Biosimilars Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Bevacizumab Biosimilars market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Bevacizumab Biosimilars market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Bevacizumab Biosimilars market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Bevacizumab Biosimilars market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Bevacizumab Biosimilars market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Bevacizumab Biosimilars value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Bevacizumab Biosimilars industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Bevacizumab Biosimilars Market Report
- Global Bevacizumab Biosimilars market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Bevacizumab Biosimilars trade, costs, and supply chains
- Bevacizumab Biosimilars market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Bevacizumab Biosimilars market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Bevacizumab Biosimilars market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Bevacizumab Biosimilars supply chain analysis
- Bevacizumab Biosimilars trade analysis, Bevacizumab Biosimilars market price analysis, and Bevacizumab Biosimilars supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Bevacizumab Biosimilars market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Cipla Limited
- Reliance lifesciences Pvt. Ltd.
- Genentech Inc.
- Fujifilm Kyowa Kirin Biologics Co. Ltd.
- Pfizer Inc.
- Abbvie Inc.
- Amgen Inc.
- F. Hoffmann-La Roche Ltd.
- Teva Pharmaceutical Industries Ltd.
- Mylan Inc.
- Daiichi Sankyo Company Limited
- Beaconpharma Ltd.
- Innovent Biologics Inc.
- STADA Arzneimittel AG
- Shanghai Henlius Biotech Inc.
- Aurobindo Pharma Ltd.
- Dr. Reddy's Laboratories Ltd.
- Biocon Ltd.
- Intas Pharmaceuticals Ltd.
- Amneal Pharmaceuticals LLC
- Samsung Bioepis Co. Ltd.
- Celltrion Inc.
- Coherus BioSciences Inc.
- Hetero Drugs Ltd.
- EirGen Pharma Ltd.
- Allergan PLC
- Apotex Inc.
- BioXpress Therapeutics SA
- Celgene Corporation Co. Ltd.
- mAbxience SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 2.1 Billion |
| Forecasted Market Value ( USD | $ 4.3 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |