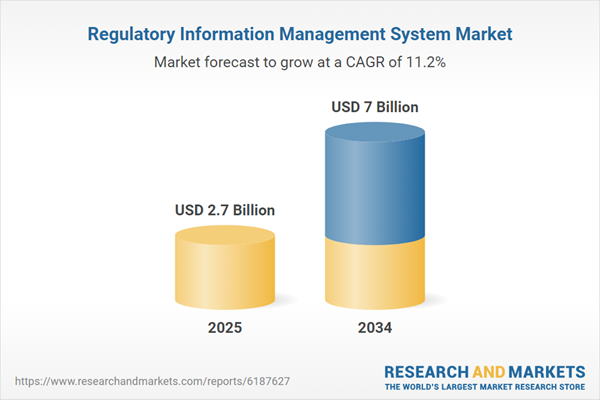
The Regulatory Information Management System (RIMS) Market is becoming an essential part of the life sciences and healthcare technology landscape, helping organizations efficiently manage product registrations, regulatory submissions, and compliance documentation across multiple geographies. These systems serve as centralized digital platforms that streamline regulatory data collection, submission lifecycle management, health authority correspondence, and compliance tracking. With increasing regulatory complexity, globalization of pharmaceutical supply chains, and growing demands for faster market access, companies are investing in RIMS to reduce errors, enhance transparency, and maintain audit readiness. RIMS platforms also support eCTD (electronic Common Technical Document) submissions, labeling control, and structured content management, enabling cross-functional collaboration among regulatory, quality, clinical, and manufacturing teams. As digital transformation reshapes how regulatory data is handled, RIMS is transitioning from a back-office tool to a strategic asset that supports efficiency, accuracy, and global regulatory agility.
The RIMS market experienced significant growth as pharmaceutical and medical device companies accelerated digital upgrades to meet global compliance requirements and regulatory submission timelines. The rollout of IDMP (Identification of Medicinal Products) standards in the EU drove adoption of structured regulatory data systems, while U.S. FDA and Health Canada expanded requirements for electronic submissions. RIMS vendors responded by enhancing integration capabilities with enterprise resource planning (ERP), clinical trial management systems (CTMS), and quality management systems (QMS), offering more comprehensive end-to-end platforms. Cloud-native RIMS solutions gained traction, allowing scalability and easier access for globally distributed teams. AI-powered features such as automated dossier compilation, intelligent metadata tagging, and predictive submission scheduling began to surface in leading platforms. Meanwhile, the growing emphasis on label lifecycle management and regional compliance led to increased adoption of region-specific modules. 2024 marked a turning point where RIMS shifted from a compliance necessity to a competitive enabler in regulatory strategy and operations.
The RIMS market is expected to see further expansion, with advanced analytics, automation, and real-time data synchronization becoming standard features. The convergence of RIMS with other regulatory and enterprise systems will support unified data environments, enabling faster and more accurate decision-making. Adoption will grow among small and mid-sized life sciences companies as SaaS-based platforms become more affordable and easier to implement. As the regulatory landscape evolves to include requirements for ESG, AI-enabled health technologies, and personalized medicine, RIMS will be adapted to manage more diverse data types and complex submission formats. Global regulatory harmonization efforts and digital-first health authority initiatives will also spur the demand for agile, interoperable RIMS platforms. Additionally, organizations will place greater emphasis on data governance, traceability, and end-to-end regulatory intelligence, turning RIMS into a core pillar of their digital regulatory infrastructure. The future of this market lies in enabling compliant, collaborative, and continuous regulatory operations on a global scale.
Key Insights: Regulatory Information Management System Market
- Cloud-based RIMS platforms are gaining momentum, offering greater scalability, real-time access, and seamless integration with other enterprise and regulatory systems.
- AI-powered automation is being introduced to support document tagging, dossier compilation, and submission timeline forecasting, improving operational efficiency and compliance readiness.
- Implementation of IDMP and SPOR (Substance, Product, Organization, Referential) standards in the EU is pushing companies to adopt structured regulatory data models within RIMS.
- Integrated labeling and artwork management tools are becoming essential features in RIMS to streamline global product lifecycle and labeling compliance.
- Growing regulatory harmonization and globalization are encouraging adoption of multi-region RIMS capabilities that support centralized data while ensuring local regulatory alignment.
- Increasing complexity of global regulatory requirements is driving the need for centralized platforms to manage data, submissions, and compliance across multiple regions and product portfolios.
- Rising pressure to accelerate product approvals and market access is prompting life sciences companies to streamline regulatory operations with automated RIMS solutions.
- Digital transformation initiatives and growing demand for structured data reporting (e.g., IDMP, eCTD) are fueling investment in next-generation regulatory systems.
- Frequent audits and the need for real-time inspection readiness are pushing organizations to adopt RIMS platforms with built-in audit trails and traceability features.
- High implementation costs, long deployment timelines, and integration complexity with legacy systems can deter small and mid-sized companies from adopting advanced RIMS platforms.
Regulatory Information Management System Market Segmentation
By Component
- Solution
- Services
By Deployment
- On-premise
- Cloud
By Enterprise Size
- Small and Medium Enterprises
- Large Enterprises
By Application
- Registration Management
- Regulatory Intelligence
- Labeling Management
- Submission Planning and Tracking Management
- Publishing
- Document Management
- Other Applications
By End-Users
- Pharmaceutical Sector
- Medical Device Sector
- Other End Users
Key Companies Analysed
- Deloitte Touche Tohmatsu Limited
- DXC Technology Company
- IQVIA Inc.
- Wipro Ltd
- Parexel International Corporation
- Korber Pharma
- Veeva Systems Inc
- Dovel Technologies Inc.
- Calyx
- ArisGlobal LLC
- DDi Inc.
- NNIT A/S
- Freyr Software Services Private Limite
- Navitas Life Sciences
- MasterControl Solutions Inc.
- Sparta Systems Inc.
- Instem PLC
- Phlexglobal Ltd.
- Ennov SA
- LORENZ Life Sciences Group
- Extedo GmbH
- Gimmal
- Dot Compliance Ltd.
- Kalypso LP
- Rimsys Inc.
- Virtify Inc.
- Glemser Technologies Corporation
- RegDesk
- AmpleLogic
- Ithos Global Inc.
Regulatory Information Management System Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Regulatory Information Management System Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Regulatory Information Management System market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Regulatory Information Management System market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Regulatory Information Management System market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Regulatory Information Management System market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Regulatory Information Management System market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Regulatory Information Management System value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Regulatory Information Management System industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Regulatory Information Management System Market Report
- Global Regulatory Information Management System market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Regulatory Information Management System trade, costs, and supply chains
- Regulatory Information Management System market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Regulatory Information Management System market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Regulatory Information Management System market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Regulatory Information Management System supply chain analysis
- Regulatory Information Management System trade analysis, Regulatory Information Management System market price analysis, and Regulatory Information Management System supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Regulatory Information Management System market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Deloitte Touche Tohmatsu Limited
- DXC Technology Company
- IQVIA Inc.
- Wipro Ltd.
- Parexel International Corporation
- Korber Pharma
- Veeva Systems Inc.
- Dovel Technologies Inc.
- Calyx
- ArisGlobal LLC
- DDi Inc.
- NNIT A/S
- Freyr Software Services Private Limite
- Navitas Life Sciences
- MasterControl Solutions Inc.
- Sparta Systems Inc.
- Instem PLC
- Phlexglobal Ltd.
- Ennov SA
- LORENZ Life Sciences Group
- Extedo GmbH
- Gimmal
- Dot Compliance Ltd.
- Kalypso LP
- Rimsys Inc.
- Virtify Inc.
- Glemser Technologies Corporation
- RegDesk
- AmpleLogic
- Ithos Global Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 2.7 Billion |
| Forecasted Market Value ( USD | $ 7 Billion |
| Compound Annual Growth Rate | 11.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |