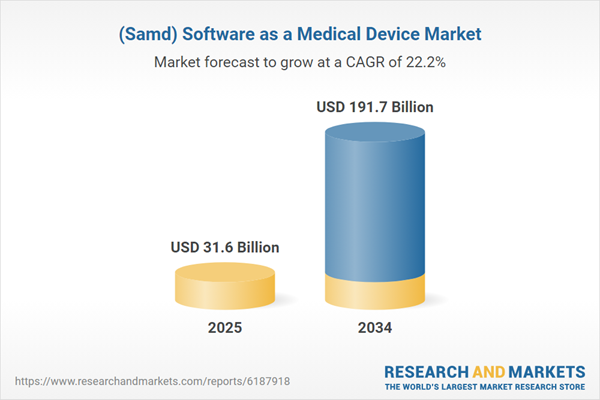
The Software as a Medical Device (SaMD) market is rapidly transforming the healthcare industry, offering innovative digital solutions that enhance diagnosis, treatment, and patient monitoring. SaMD refers to software applications that function independently of traditional hardware medical devices, providing real-time analysis, clinical decision support, and remote patient monitoring. These solutions are widely used in telemedicine, diagnostics, imaging, and personalized treatment plans, making healthcare more efficient and accessible. The growing adoption of artificial intelligence (AI) and machine learning (ML) in medical software is significantly improving predictive analytics and patient outcomes. Regulatory bodies such as the FDA, EMA, and other international health authorities are increasingly focusing on establishing comprehensive guidelines for the approval and safe use of SaMD solutions. As healthcare providers embrace digital transformation, the demand for software-driven medical solutions continues to grow, particularly with the expansion of remote healthcare and digital therapeutics. The increasing prevalence of chronic diseases, coupled with a rising aging population, is further fueling the adoption of SaMD. As technological advancements continue, the market is expected to witness exponential growth, with companies investing in cybersecurity, interoperability, and compliance to enhance patient safety and regulatory acceptance.
The SaMD market experienced remarkable advancements driven by regulatory developments, AI integration, and the expansion of digital health ecosystems. Regulatory agencies strengthened compliance frameworks, ensuring that medical software meets rigorous safety and efficacy standards. AI-powered SaMD applications, particularly in radiology and diagnostics, gained widespread adoption, enabling faster and more accurate disease detection. Telehealth solutions powered by SaMD also saw a surge in usage, as healthcare providers sought more efficient ways to deliver remote patient care. The pharmaceutical industry increasingly integrated SaMD solutions into drug development and clinical trials, leveraging real-world data for improved patient monitoring and personalized treatment approaches. Additionally, cybersecurity emerged as a critical focus area, with companies investing in robust encryption and threat detection systems to safeguard patient data. Emerging economies, particularly in Asia-Pacific and Latin America, experienced heightened SaMD adoption, supported by digital health initiatives and increasing investments in healthcare infrastructure. With continuous regulatory refinements and ongoing technological innovations, 2024 proved to be a pivotal year in advancing SaMD’s capabilities and expanding its role in modern healthcare.
The SaMD market is expected to witness further evolution, driven by advancements in AI, cloud computing, and real-time data analytics. AI-driven diagnostic software will continue to enhance clinical decision-making, reducing diagnostic errors and improving treatment accuracy. The integration of blockchain technology for secure data sharing and interoperability will become a key focus, allowing seamless communication across healthcare systems while ensuring data integrity. Regulatory harmonization efforts across major markets will help streamline approval processes, fostering faster adoption of innovative medical software solutions. Additionally, personalized medicine will play a crucial role in shaping the future of SaMD, enabling tailored treatments based on genetic and real-time health data. Emerging markets will see increased investments in digital health, with governments prioritizing healthcare accessibility and technology-driven solutions. As the industry moves toward fully connected healthcare ecosystems, interoperability and cybersecurity will remain top priorities, ensuring seamless, secure, and efficient deployment of SaMD across healthcare settings. With continuous innovation and regulatory alignment, the SaMD market is poised for long-term expansion, transforming the future of digital healthcare.
Key Insights: (Samd) Software As A Medical Device Market
- Integration of AI and Machine Learning in SaMD: AI-powered SaMD solutions are revolutionizing healthcare by improving diagnostic accuracy, predicting disease progression, and enhancing clinical decision support. Machine learning algorithms enable personalized treatment recommendations by analyzing vast amounts of patient data in real time. In radiology, AI-driven imaging software is reducing diagnostic errors and expediting disease detection. The continued evolution of AI in SaMD is expected to make healthcare more predictive and preventive, significantly improving patient care and treatment outcomes.
- Rise of Regulatory Frameworks and Compliance Standards: As SaMD adoption increases, regulatory bodies worldwide are establishing stricter compliance standards to ensure patient safety and data security. The FDA, EMA, and other international regulatory agencies are refining approval processes to address emerging technologies like AI-driven diagnostics and remote patient monitoring solutions. These evolving frameworks provide clearer guidelines for manufacturers, fostering market growth while ensuring software reliability. Compliance with these regulations will be a crucial factor for companies aiming to expand their presence in the SaMD market.
- Growing Adoption of Telehealth and Remote Monitoring: The demand for remote healthcare solutions has surged, accelerating the adoption of SaMD applications in telehealth and patient monitoring. With an increasing aging population and rising cases of chronic diseases, remote monitoring tools integrated with SaMD help healthcare providers track patient conditions in real-time, reducing hospital visits. This trend is further supported by government policies promoting digital healthcare infrastructure, making telemedicine a permanent component of healthcare delivery.
- Expansion of Personalized Medicine and Digital Therapeutics: SaMD is playing a crucial role in advancing personalized medicine by enabling software-driven treatment plans based on patient-specific data. Digital therapeutics, which use SaMD to deliver evidence-based therapeutic interventions, are gaining traction for managing chronic conditions like diabetes and mental health disorders. With advancements in genomics and wearable technology, SaMD solutions are becoming more tailored to individual patient needs, enhancing treatment precision and overall healthcare efficiency.
- Cybersecurity and Data Privacy Concerns: As SaMD applications rely heavily on cloud computing and real-time data exchange, cybersecurity threats pose a significant challenge. The risk of data breaches, ransomware attacks, and unauthorized access to sensitive patient information remains a major concern for healthcare providers and regulatory agencies. Ensuring robust data encryption, regulatory compliance, and continuous security monitoring will be critical for maintaining patient trust and protecting sensitive medical data in the rapidly evolving digital healthcare landscape.
(Samd) Software As A Medical Device Market Segmentation
By Device Type
- Wearable Device
- PCs and Laptop
- Smartphone and Tablets
By Deployment Method
- Cloud-Based
- On-Premise
By Application
- Diagnostic
- Clinical Management
Key Companies Analysed
- Apple Inc.
- McKesson Corporation
- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Siemens Healthcare GmbH
- GE HealthCare Technologies Inc.
- Roche Diagnostics
- 3M Health Care
- Cerner Corporation
- Illumina Inc.
- Philips Medical Systems Ltd.
- Dexcom Inc.
- Sysmex Corporation
- Carl Zeiss Meditec AG
- Nihon Kohden Corporation
- Zhlke Group
- Viz ai Inc.
- Paragon Biosciences LLC
- S3 Connected Health
- Arterys Inc.
- BrightInsight Inc.
- Agfa HealthCare Corp.
- iSchemaView Inc.
- VitalConnect Inc.
- Volpara Health Technologies
- Tietronix Software Inc.
- Velentium LLC
- Greenfinch Technology Ltd.
- MaxQ AI Ltd.
- IDx Technologies Inc.
(Samd) Software As A Medical Device Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
(Samd) Software As A Medical Device Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - (Samd) Software As A Medical Device market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - (Samd) Software As A Medical Device market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - (Samd) Software As A Medical Device market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - (Samd) Software As A Medical Device market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - (Samd) Software As A Medical Device market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the (Samd) Software As A Medical Device value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the (Samd) Software As A Medical Device industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the (Samd) Software As A Medical Device Market Report
- Global (Samd) Software As A Medical Device market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on (Samd) Software As A Medical Device trade, costs, and supply chains
- (Samd) Software As A Medical Device market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- (Samd) Software As A Medical Device market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term (Samd) Software As A Medical Device market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and (Samd) Software As A Medical Device supply chain analysis
- (Samd) Software As A Medical Device trade analysis, (Samd) Software As A Medical Device market price analysis, and (Samd) Software As A Medical Device supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest (Samd) Software As A Medical Device market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Apple Inc.
- McKesson Corporation
- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Siemens Healthcare GmbH
- GE HealthCare Technologies Inc.
- Roche Diagnostics
- 3M Health Care
- Cerner Corporation
- Illumina Inc.
- Philips Medical Systems Ltd.
- Dexcom Inc.
- Sysmex Corporation
- Carl Zeiss Meditec AG
- Nihon Kohden Corporation
- Zhlke Group
- Viz ai Inc.
- Paragon Biosciences LLC
- S3 Connected Health
- Arterys Inc.
- BrightInsight Inc.
- Agfa HealthCare Corp.
- iSchemaView Inc.
- VitalConnect Inc.
- Volpara Health Technologies
- Tietronix Software Inc.
- Velentium LLC
- Greenfinch Technology Ltd.
- MaxQ AI Ltd.
- IDx Technologies Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 31.6 Billion |
| Forecasted Market Value ( USD | $ 191.7 Billion |
| Compound Annual Growth Rate | 22.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |