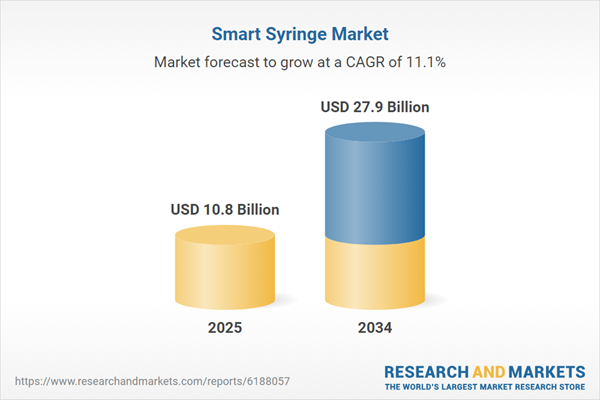
The smart syringe market is gaining prominence as healthcare providers worldwide seek safer, more efficient, and technologically advanced solutions for drug delivery and vaccination. Smart syringes are designed to prevent needle reuse, minimize needlestick injuries, and enable precise dosage administration. Equipped with safety mechanisms, auto-disable features, and in some cases, digital tracking capabilities, these syringes have found growing utility in vaccination drives, chronic disease management, and hospital settings. As regulatory authorities emphasize patient safety and as global health programs increase immunization efforts, the need for smarter injection devices has surged. These devices also align well with digital health trends by offering connectivity to electronic medical records (EMR) and enabling better inventory control and patient compliance tracking. With an aging population and rising incidence of diseases like diabetes, hepatitis, and cancer, the relevance of smart syringes in both developed and developing healthcare systems is expected to rise significantly in the coming years.
The smart syringe market experienced notable momentum, largely driven by mass immunization campaigns and an intensified global focus on injection safety. Several health agencies and governments expanded the use of auto-disable syringes in routine vaccination programs, particularly in regions with high infectious disease burdens. Manufacturers introduced smart syringes embedded with RFID and barcode scanning technologies, enabling seamless integration with hospital data systems and enhancing traceability. Hospitals began leveraging smart syringes with pre-set dosing systems for oncology and critical care to reduce medication errors. Partnerships between syringe manufacturers and healthcare IT providers also grew, focusing on connected syringes that could log injection time, patient data, and drug type directly into EMR platforms. In response to mounting concerns about medical waste, companies also began piloting biodegradable smart syringe components, aligning with environmental sustainability goals in the healthcare sector. These innovations positioned smart syringes as a key component of the broader smart healthcare ecosystem, complementing efforts in digital therapeutics and connected diagnostics.
The smart syringe market is expected to evolve further with enhanced automation, increased adoption in home healthcare, and integration with AI-driven drug delivery monitoring platforms. The home-use segment, particularly for patients with chronic illnesses requiring regular injections, is likely to see significant expansion. Next-generation smart syringes may include real-time monitoring of adherence, dosage feedback, and alerts for missed doses - all connected via mobile applications. Regulatory agencies are expected to mandate stricter compliance with needle safety and single-use requirements, prompting wider procurement of smart syringes across public and private sectors. The market will also benefit from advancements in manufacturing technologies that reduce cost and enable large-scale production of smart features without compromising usability. However, to unlock this potential, healthcare systems will need to invest in training, digital infrastructure, and cross-platform interoperability to fully leverage the benefits of these intelligent devices. As such, the smart syringe market is not just a medical device segment but a key enabler of future-ready healthcare systems.
Key Insights: Smart Syringe Market
- Adoption of Auto-Disable Syringes in Immunization Programs: Governments and global health agencies are deploying auto-disable syringes for vaccination to prevent reuse, reduce infection transmission, and improve compliance with WHO safety standards in developing nations.
- Integration of RFID and Barcode Scanning Technologies: Smart syringes are being embedded with digital identifiers to enable seamless data logging into hospital records, enhancing drug traceability and reducing risks of administration errors.
- Growth of Connected Syringes in Chronic Disease Management: Devices with built-in connectivity to mobile apps and EMRs are improving medication adherence and enabling remote patient monitoring in conditions like diabetes and rheumatoid arthritis.
- Rising Focus on Eco-Friendly Syringe Materials: Manufacturers are developing smart syringes with recyclable or biodegradable materials to address growing concerns over medical waste and support sustainability in healthcare supply chains.
- Expansion of Smart Syringes in Home Healthcare Settings: User-friendly smart syringes designed for home use are gaining popularity, especially among patients needing self-administered therapies, supported by digital training tools and adherence reminders.
- Rising Incidence of Needleborne Infections: The global push to eliminate transmission risks associated with reused or mishandled syringes is driving strong demand for safety-engineered, smart injection devices.
- Increasing Global Vaccination Initiatives: Large-scale immunization programs led by governments and international health bodies are prompting widespread adoption of smart syringes that ensure safety, efficiency, and dose accuracy.
- Technological Advancements in Drug Delivery: Innovations in smart injection technology, including connectivity and auto-dosing, are improving healthcare delivery while meeting the growing expectations for digitized and personalized treatment.
- Stringent Regulatory Mandates on Syringe Reuse Prevention: Regulatory guidelines promoting the use of auto-disable and safety syringes in clinical and public health settings are accelerating their adoption in both developed and emerging markets.
- High Cost and Access Barriers in Low-Income Regions: The advanced features and technology integration in smart syringes lead to higher costs, making widespread adoption challenging in resource-limited settings where basic healthcare access is still a struggle.
Smart Syringe Market Segmentation
By Product
- Auto-Disable Syringes
- Active Safety Syringes
- Passive Safety Syringes
By Age Group
- Pediatrics
- Adults
By Application
- Drug Delivery
- Vaccination
- Blood Specimen Collection
By End Users
- Hospitals and Health Maintenance Organizations (HMOs)
- Diabetic Patients
- Family Practices
- Psychiatrics
- Other End Users
Key Companies Analysed
- Becton Dickinson and Company
- Gerresheimer AG
- Baxter International Inc.
- Terumo Corporation
- Braun Melsungen AG
- Cardinal Health Inc.
- Smiths Medical Inc
- Parker-Hannifin Corporation
- Retractable Technologies Inc.
- AdvaCare Pharma
- Numedico Technologies Pty Ltd.
- Merit Medical Systems Inc.
- Nipro Corporation
- Revolutions Medical Corporation
- Sharps Technology
- Unilife Corporation
- West Pharmaceutical Services Inc.
- Schott AG
- Vetter Pharma International GmbH
- Catalent Inc.
- Agilent Technologies Inc.
- Shimadzu Corporation
- Thermo Fisher Scientific Inc.
- Trajan LEAP PAL Parts + Consumables
- Clayens-np
- Haselmeier GmbH
- Owen Mumford Ltd.
- Ypsomed Holding AG
- Weigao Group
- Jiangsu Huida Medical Instruments Co. Ltd.
- Shandong Weigao Group Medical Polymer Co. Ltd.
- Zibo Minkang Pharmaceutical Packing Co. Ltd.
Smart Syringe Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Smart Syringe Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Smart Syringe market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Smart Syringe market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Smart Syringe market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Smart Syringe market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Smart Syringe market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Smart Syringe value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Smart Syringe industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Smart Syringe Market Report
- Global Smart Syringe market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Smart Syringe trade, costs, and supply chains
- Smart Syringe market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Smart Syringe market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Smart Syringe market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Smart Syringe supply chain analysis
- Smart Syringe trade analysis, Smart Syringe market price analysis, and Smart Syringe supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Smart Syringe market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Becton Dickinson and Company
- Gerresheimer AG
- Baxter International Inc.
- Terumo Corporation
- Braun Melsungen AG
- Cardinal Health Inc.
- Smiths Medical Inc.
- Parker-Hannifin Corporation
- Retractable Technologies Inc.
- AdvaCare Pharma
- Numedico Technologies Pty Ltd.
- Merit Medical Systems Inc.
- Nipro Corporation
- Revolutions Medical Corporation
- Sharps Technology
- Unilife Corporation
- West Pharmaceutical Services Inc.
- Schott AG
- Vetter Pharma International GmbH
- Catalent Inc.
- Agilent Technologies Inc.
- Shimadzu Corporation
- Thermo Fisher Scientific Inc.
- Trajan LEAP PAL Parts + Consumables
- Clayens-np
- Haselmeier GmbH
- Owen Mumford Ltd.
- Ypsomed Holding AG
- Weigao Group
- Jiangsu Huida Medical Instruments Co. Ltd.
- Shandong Weigao Group Medical Polymer Co. Ltd.
- Zibo Minkang Pharmaceutical Packing Co. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 10.8 Billion |
| Forecasted Market Value ( USD | $ 27.9 Billion |
| Compound Annual Growth Rate | 11.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 32 |