Speak directly to the analyst to clarify any post sales queries you may have.
Establishing the Framework: A Comprehensive Overview of Cranial Repair Products Market Dynamics and Strategic Imperatives
The cranial repair products arena encompasses an intricate network of technological innovation, clinical practice evolution, and regulatory oversight. Stakeholders ranging from medical device engineers to neurosurgeons collaborate in a high-stakes environment where safety, biocompatibility, and performance are paramount. Implants and instruments designed for cranial reconstruction must align with stringent quality standards, while also adapting to diverse procedural needs that span trauma, reconstructive work, neurosurgery, and cosmetic enhancement. As materials science advances, manufacturers leverage ceramic composites, polyetheretherketone variations, polymethylmethacrylate formulations, and titanium alloys to meet these multifaceted demands. Concurrently, evolving surgical techniques emphasize minimally invasive protocols, image-guided navigation, and patient-specific customization, driving product refinement and process optimization.This summary distills the core dynamics shaping the market, mapping the interplay of supply chain complexities, stakeholder expectations, and competitive strategies. It illuminates how direct sales, distributor networks, and both B2B and B2C e-commerce channels intersect to deliver critical solutions to ambulatory surgical centers, major hospitals, specialty clinics, and research institutes. By setting a clear analytical foundation, this introduction frames subsequent exploration of transformative trends, tariff impacts, segmentation insights, regional variations, leading company profiles, actionable recommendations, and the robust methodological approach underpinning the findings.
Navigating Emerging Disruptors: Uncovering the Critical Technological, Clinical and Regulatory Shifts Reshaping the Future of Cranial Repair Products
Technological breakthroughs are reshaping cranial repair with unprecedented speed. Additive manufacturing techniques now enable patient-specific implants that conform precisely to three-dimensional anatomical scans, reducing operation times and improving clinical outcomes. At the same time, advanced biomaterials-ranging from next-generation polyetheretherketone composites to bioactive ceramics-offer enhanced osseointegration, infection resistance, and mechanical resilience. These material innovations are complemented by digital planning platforms that facilitate virtual surgical rehearsal, thereby elevating procedural accuracy and reducing intraoperative risk.Clinically, the shift toward minimally invasive approaches is redefining instrument requirements. Drills optimized for reduced vibration, precision saws with enhanced blade geometries, and navigation-enabled tools are becoming indispensable. As surgeons integrate robotic assistance and augmented reality overlays, the instrument portfolio adapts to new ergonomic and digital integration demands.
Regulatory evolution further accelerates change. Streamlined approval pathways for custom devices, updated guidance on sterilization protocols, and emerging reimbursement frameworks for advanced therapies are reshaping market entry strategies. Together, these technological, clinical, and regulatory vectors converge to create a landscape of rapid innovation, demanding agility and foresight from manufacturers and healthcare providers alike.
Assessing the Ripple Effects: How 2025 United States Tariffs Are Transforming Supply Chains, Cost Structures and Strategic Planning in Cranial Repair
The imposition of new United States tariffs in 2025 introduces significant cost implications for cranial repair supply chains. Raw materials such as titanium alloys and specialty polymers now face additional duties, leading manufacturers to reassess sourcing strategies and inventory approaches. Some device producers are negotiating longer-term contracts with domestic suppliers or exploring localized manufacturing partnerships to mitigate tariff exposure. This strategic pivot is prompting consolidation of high-value components into modular kits that can be assembled closer to end users, reducing cross-border freight complexities and import duty burdens.Meanwhile, cost pressures ripple through distribution pathways. Direct sales teams and third-party distributors must revise pricing models, balancing margin retention with competitive positioning. E-commerce platforms serving both hospital procurement professionals and private clinics are adjusting fulfillment strategies to optimize landed costs. In response, leading stakeholders are engaging in scenario planning and cost modeling to preserve financial resilience while maintaining product accessibility. As these tariff-driven dynamics unfold, companies that proactively reevaluate supplier networks and embrace flexible sourcing will gain a decisive edge in navigating the altered trade environment.
Decoding Market Dynamics Through Segmentation of Product Types, Materials, Applications, End User Profiles and Distribution Paths in Cranial Repair
Insight into the cranial repair market emerges from an integrated examination of product variants by type. Implant portfolios encompass cranial flaps designed to mimic native bone contours, mesh constructs that facilitate tissue stabilization, and plates engineered for rigid fixation. Complementing these offerings, surgical instruments-ranging from precision drills to stealth-engineered saws-ensure procedural consistency and efficiency.Equally crucial are material distinctions. Bioinert ceramics deliver compressive strength for load-bearing applications, while high-performance polymers such as PEEK and polymethylmethacrylate provide versatile options for contouring and in vivo compatibility. Titanium remains a stalwart choice for its strength-to-weight ratio and long-standing clinical track record. These materials intersect with specialized application fields, where cosmetic revisions demand refined aesthetics, neurosurgical interventions require structural reliability, reconstructive efforts emphasize tissue integration, and trauma protocols prioritize rapid stabilization.
End users span ambulatory surgical centers seeking cost-effective, streamlined procedural kits, large hospital systems requiring comprehensive device libraries, research institutes driving next-generation innovation, and specialty clinics focusing on niche cosmetic and reconstructive therapies. Distribution mechanisms range from direct engagement by field teams to established distributor networks and e-commerce channels that cater to business customers and healthcare professionals alike. Through this multi-dimensional lens, market complexity becomes navigable, revealing precise levers for targeted growth and optimization.
Unearthing Regional Drivers: Comprehensive Perspectives on Market Behavior Across Americas, Europe Middle East and Africa, and Asia-Pacific in Cranial Repair
Regional analysis reveals distinct market drivers across diverse geographies. In the Americas, robust investment in healthcare infrastructure and a mature reimbursement environment support widespread adoption of advanced implants and digitally integrated instruments. Medical centers focus on multidisciplinary collaboration, fostering rapid clinical validation of novel materials and precision-guided surgical solutions.Meanwhile, Europe, Middle East and Africa present a mosaic of regulatory frameworks and purchasing behaviors. Western European markets emphasize compliance with stringent safety standards and prioritize long-term clinical outcomes, whereas emerging economies in the Middle East and Africa seek cost-effective solutions that maintain essential performance criteria. Public-private partnerships and regional innovation hubs are accelerating the adaptation of digital planning tools and additive manufacture, paving the way for localized production models and knowledge transfer initiatives.
Asia-Pacific demonstrates dynamic momentum driven by expanding neurosurgical centers, rising trauma care demand, and targeted government funding for medical device research. Local manufacturers are forging alliances to co-develop implants using indigenous material resources, while international suppliers tailor logistics and training programs to support rapid market entry. Collectively, these regional forces shape a heterogeneous landscape where strategic footprint allocation and adaptive engagement models are essential.
Illuminating Leading Players: In-Depth Analysis of Strategic Positioning and Competitive Edge Among Key Cranial Repair Industry Companies
Leading companies in the cranial repair domain distinguish themselves through differentiated innovation pipelines. Some have invested heavily in additive manufacturing partnerships to offer customizable implant geometries aligned with patient-specific CT data. Others focus on proprietary polymer formulations that balance radiolucency with mechanical performance, enabling both neurosurgical and imaging requirements to be met seamlessly.Strategic collaborations with academic research institutes underpin several firms’ competitive edge. Joint development agreements are yielding bioactive coatings that promote osteogenic integration, while co-funded clinical trials validate next-generation mesh constructs for reconstructive use. These partnerships not only accelerate time to market but also enhance regulatory positioning through evidence-based safety profiles.
In the instrument space, a subset of companies excels at integrating digital telemetry into drills and saws, providing real-time feedback on torque, blade wear, and procedural milestones. This data-driven approach supports post-market surveillance and continuous product improvement. Complementing technical advancements, nimble manufacturers are forging distribution alliances with global logistics providers and specialized e-commerce platforms to ensure rapid fulfillment and end-user training, further solidifying their leadership positions.
Crafting Strategic Pathways: Actionable Recommendations Empowering Industry Leadership to Navigate Opportunities and Challenges in Cranial Repair
Industry leaders should advance local manufacturing initiatives to navigate supply chain volatility and mitigate tariff impacts. Establishing regional production centers near core demand clusters promotes faster lead times and reduces dependency on cross-border logistics. Concurrently, diversifying supplier networks for critical materials such as titanium and high-performance polymers minimizes exposure to single-source constraints. Building robust procurement frameworks with dual sourcing agreements and strategic inventory buffers will support uninterrupted clinical workflows.In parallel, leaders must accelerate digital integration across both product design and instrument utilization. Embracing virtual preoperative planning platforms and instrument telemetry systems enhances procedural precision and generates actionable post-market data. Collaboration with healthcare providers to design training modules and simulation-based curricula ensures optimal instrument adoption and long-term clinical success. By aligning these initiatives with evolving regulatory pathways and reimbursement policies, organizations can translate innovation into sustained competitive advantage.
Revealing the Robust Research Framework: Methodological Approaches Underpinning Precision and Rigor in Cranial Repair Market Analysis
The research methodology integrates comprehensive secondary research with targeted primary engagements. Initial desk analysis encompassed review of peer-reviewed journals, regulatory filings, and patents to map technological trajectories and material innovations. This foundation informed the development of structured interview protocols for clinical experts, device engineers, procurement professionals, and regulatory authorities.Primary interviews were conducted across multiple regions to capture nuanced perspectives on adoption hurdles, training requirements, and supply chain priorities. A Delphi approach refined consensus around emerging best practices, while iterative data triangulation ensured alignment between quantitative trends and qualitative insights. All inputs underwent rigorous validation through cross-verification with publicly available clinical trial outcomes and manufacturing data.
To safeguard analytical integrity, the study employed a multi-tiered quality assurance process. Internal review committees assessed methodological adherence, data consistency checks were automated, and final findings were audited against real-world case studies. This robust framework ensures that the research delivers reliable, actionable intelligence to inform strategic decision-making in the cranial repair market.
Synthesizing Critical Insights: Concluding Perspectives on Market Evolution, Strategic Imperatives and Future Readiness in Cranial Repair Products
Drawing together technological evolution, regulatory shifts, and market segmentation reveals a landscape defined by both challenge and opportunity. The convergence of advanced biomaterials, patient-specific manufacturing, and digital surgical integration underscores a paradigm shift toward personalized cranial repair solutions. At the same time, tariff pressures and regional nuances demand strategic agility, compelling stakeholders to adapt sourcing strategies and go-to-market models.By aligning competitive positioning with targeted segmentation insights-spanning implant variants, material profiles, clinical applications, end user dynamics, and distribution channels-organizations can chart growth pathways tailored to regional and clinical imperatives. Coupled with actionable leadership recommendations and a rigorous methodological foundation, this synthesis equips decision-makers with a holistic view of the market. Harnessing these insights will be essential for sustaining innovation leadership and achieving enduring clinical impact in the cranial repair product domain.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Implants
- Cranial Flaps
- Cranial Mesh
- Cranial Plates
- Instruments
- Drills
- Saws
- Implants
- Material
- Ceramic
- Peek
- Polymethylmethacrylate
- Titanium
- Application
- Cosmetic
- Neurosurgery
- Reconstructive
- Trauma
- End User
- Ambulatory Surgical Centers
- Hospitals
- Research Institutes
- Specialty Clinics
- Distribution Channel
- Offline
- Online
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Johnson and Johnson Services Inc.
- Stryker Corporation
- adeor Medical AG
- B. Braun SE
- Bioplate
- Craniotech
- evonos GmbH & Co. KG
- Integra LifeSciences Corporation
- JEIL MEDICAL CORPORATION
- KLS Martin SE & Co. KG
- Kontour(Xi'an) Medical Technology Co., Ltd.
- Matrix Surgical USA
- Medartis AG
- MEDICON eG
- Medtronic PLC
- OsteoMed by Acumed LLC
- Oxford Performance Materials, Inc.
- pro med instruments GmbH
- Renishaw plc
- TeDan Surgical Innovations, Inc.
- Xilloc Medical Int B.V.
- Zimmer Biomet Holdings, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Cranial Repair Products market report include:- Johnson and Johnson Services Inc.
- Stryker Corporation
- adeor Medical AG
- B. Braun SE
- Bioplate
- Craniotech
- evonos GmbH & Co. KG
- Integra LifeSciences Corporation
- JEIL MEDICAL CORPORATION
- KLS Martin SE & Co. KG
- Kontour(Xi'an) Medical Technology Co., Ltd.
- Matrix Surgical USA
- Medartis AG
- MEDICON eG
- Medtronic PLC
- OsteoMed by Acumed LLC
- Oxford Performance Materials, Inc.
- pro med instruments GmbH
- Renishaw plc
- TeDan Surgical Innovations, Inc.
- Xilloc Medical Int B.V.
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | October 2025 |
| Forecast Period | 2025 - 2032 |
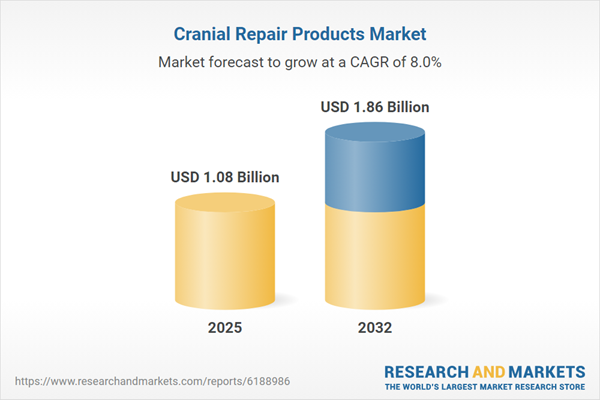
| Estimated Market Value ( USD | $ 1.08 Billion |
| Forecasted Market Value ( USD | $ 1.86 Billion |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |