Speak directly to the analyst to clarify any post sales queries you may have.
A strategic overview presenting the clinical, consumer, regulatory, and innovation themes that are reshaping product development and procurement priorities
The instant cold pack landscape sits at the intersection of clinical practice, consumer-first aid behaviors, and materials innovation. Over recent years, end users ranging from sports professionals and recreational athletes to emergency responders and household consumers have relied on instant cold packs for rapid, portable cooling when conventional refrigeration is unavailable. Clinicians and care providers value the immediacy and convenience of these single‑use or reusable devices for acute injury management, postoperative care, and pain mitigation, while manufacturers have intensified efforts to differentiate through chemical formulations, packaging materials, and environmental positioning.In parallel, industry stakeholders are responding to evolving regulatory attention on chemical safety, biodegradability of packaging, and transportation controls for reactive nitrogen‑based compounds. These pressures have accelerated R&D investment in alternative chemistries and fabrications that maintain performance while reducing risk and environmental footprint. As a result, product roadmaps increasingly balance clinical efficacy with lifecycle considerations, patient comfort, and retail presentation. Strategic players are therefore aligning product portfolios to meet clinician preferences for reliability and consumer expectations for sustainability, all while navigating complex supply chain constraints and compliance requirements.
This introduction frames the operative themes explored in the remainder of the summary: how shifts in chemistry, packaging, application contexts, and trade policy are reshaping competitive dynamics, procurement strategies, and innovation priorities for the instant cold pack sector.
How chemistry innovation, packaging sustainability, shifting end‑user expectations, and regulatory complexity are fundamentally altering competitive dynamics and product roadmaps
The instant cold pack market is experiencing transformative shifts driven by material science advances, heightened environmental expectations, and evolving care pathways. Innovations in biodegradable packaging and fabric‑coated pack technologies are moving from niche experiments into commercially viable alternatives, prompting manufacturers to reassess design priorities and cost structures. Concurrently, advances in chemical formulation are reducing reliance on more hazardous reactive nitrates and improving thermal longevity, which in turn supports broader application in clinical and field environments.Consumer behavior is another potent catalyst. Greater awareness of sustainability and demand for portable, reliable first‑aid solutions have elevated standards for both disposability and reusability. Health and wellness trends, including the growth of amateur and organized sports participation, have increased routine usage and created new channels for distribution through fitness retailers and team outfitting services. Meanwhile, institutional buyers such as hospitals, clinics, and emergency medical services are prioritizing product safety, chain‑of‑custody for supplies, and compatibility with clinical protocols, which encourages suppliers to standardize product specifications across procurement categories.
Finally, regulatory scrutiny and transport safety requirements have introduced a layer of compliance complexity that alters sourcing decisions and inventory management. These cumulative shifts are compelling both incumbents and new entrants to pursue integrated strategies-combining chemistry innovation, packaging redesign, and robust regulatory engagement-to secure lasting differentiation and market access.
The cumulative impact of United States tariff measures on sourcing resilience, landed costs, supplier diversification, and product redesign imperatives
In 2025, tariff developments out of the United States have had a measurable influence on supply chains, sourcing strategies, and procurement behavior across the instant cold pack ecosystem. Import duties and associated compliance measures have increased landed costs for certain imported components, prompting buyers and manufacturers to evaluate alternative suppliers, nearshoring options, and localized manufacturing capabilities. These actions have not only reshaped supplier negotiation dynamics but have also influenced inventory planning and contract durations as organizations seek to mitigate volatility.As a consequence of the tariff environment, some manufacturers have accelerated supplier diversification to reduce concentration risk and to maintain consistent access to key raw materials and packaging substrates. This has led to expanded relationships with regional suppliers across the Americas and Asia‑Pacific, and an uptick in dual‑sourcing strategies that combine cost, lead‑time reliability, and regulatory familiarity. In parallel, procurement teams have prioritized transparency in total landed cost calculations, which now routinely incorporate duties, compliance costs, and end‑to‑end logistical contingencies.
Tariff pressures have also stimulated innovation in material selection and product design. Firms have sought to substitute higher‑duty inputs with lower‑tariff alternatives or to reconfigure packaging and chemical formulations in ways that reduce tariff exposure without sacrificing performance. Moreover, regulatory and customs complexity has increased demand for compliance‑focused services, including classification audits and trade advisory support, which are becoming integral to strategic supply‑chain planning for market participants.
Insightful segmentation synthesis revealing how product type, chemistry, packaging, application, and end‑user distinctions drive differentiated product strategies and procurement choices
A granular understanding of product and end‑use segmentation reveals where innovation and demand converge within the instant cold pack landscape. Based on Product Type, market participants differentiate between Disposable and Reusable formats, each addressing distinct use cases and procurement channels; disposable packs continue to serve single‑event applications and retail convenience, while reusable systems target recurring clinical or athletic use with a focus on durability and lifecycle costs. Based on Chemical Composition, manufacturers work across Ammonium Nitrate‑Based, Calcium Ammonium Nitrate‑Based, and Urea‑Based formulations, with each chemistry presenting trade‑offs in reactivity, thermal profile, transport classification, and perceived safety across institutional buyers.Based on Packaging Material, the sector is experimenting with Biodegradable Materials, Fabric‑coated Packs, Nylon, and Polyethylene (PE), balancing product integrity, shelf stability, and environmental credentials; fabric coatings and biodegradable substrates are gaining traction where disposal regulation or consumer preferences favor lower environmental impact. Based on Application, instant cold packs are specified for Burns, Bruises & Skin‑Related Trauma, Chronic Pain, Injury Management & Sports Medicine, and Post‑Procedural & Surgical Care, and these application contexts inform design priorities such as conformability, duration of effect, and sterility considerations for clinical settings. Based on End User, the ecosystem spans Consumer & Household, Hospitals & Clinics, Military & Public Safety, Sports & Fitness, and Veterinary & Animal Care; within Hospitals & Clinics there is further differentiation across Ambulatory Care & Clinics, Dental & Oral Surgery Practices, Emergency Medical Services (EMS), and Physical Therapy & Rehab Centers, while Sports & Fitness segments into Gyms & Fitness Studios, Outdoor & Adventure Sports, and Schools & Youth Sports.
Taken together, these segmentation layers guide product development and commercialization choices. For example, reusable formats combined with urea‑based chemistries and fabric‑coated packs present compelling propositions for physical therapy settings where repeated use and patient comfort are critical, whereas disposable ammonium nitrate packs paired with polyethylene packaging remain prevalent for consumer first aid kits due to simplicity and cost. Recognizing the cross‑cutting demands of safety, regulatory compliance, and sustainability will be essential for companies sculpting portfolios aligned to specific end‑user pathways.
Regional dynamics and regulatory expectations across the Americas, Europe, Middle East & Africa, and Asia‑Pacific that shape distribution, compliance, and product differentiation
Regional dynamics significantly influence product positioning, regulatory compliance, and supply‑chain architecture across the instant cold pack sector. In the Americas, demand is shaped by a blend of consumer retail penetration, strong institutional procurement channels, and an emphasis on rapid availability for sports and emergency services. Manufacturers serving this region prioritize distribution agility, compliance with domestic transport and chemical handling standards, and packaging that meets consumer expectations for convenience and safety. Meanwhile, in Europe, Middle East & Africa, regulatory complexity and sustainability mandates are more pronounced in several markets, driving manufacturers toward higher environmental performance in materials and clearer labeling for chemical content, which in turn affects product development timelines and market entry requirements.In the Asia‑Pacific region, diverse manufacturing bases and a broad spectrum of end‑user requirements create both opportunity and complexity. Production capabilities in Asia‑Pacific support high‑volume manufacturing and enable cost‑competitive supply to global markets, but buyers often evaluate suppliers for compliance with international safety standards and for the ability to deliver consistent quality across geographies. Across all regions, geopolitical factors and regional trade arrangements influence routing decisions and cost structures, prompting firms to tailor go‑to‑market approaches that combine regional distribution partnerships, localized packaging variations, and targeted clinical evidence supporting use in specific healthcare settings.
These regional insights suggest that a one‑size‑fits‑all product strategy is unlikely to achieve optimal penetration; instead, successful firms align product attributes, regulatory dossiers, and distribution models to the distinct priorities of the Americas, Europe, Middle East & Africa, and Asia‑Pacific markets.
How competitive positioning, strategic partnerships, and innovation in chemistry and packaging determine commercial advantage and pathway to larger institutional contracts
Competitive dynamics in the instant cold pack space are driven by a mix of brand recognition, manufacturing scale, innovation in formulation and packaging, and the ability to meet regulatory and institutional procurement criteria. Leading players prioritize investments in R&D to optimize chemical performance and reduce transportation and handling constraints, while others focus on vertical integration to secure critical inputs and control quality across batch production. Strategic partnerships with material suppliers, testing laboratories, and logistics providers enable faster iteration cycles and improved responsiveness to regulatory changes.Market entrants and smaller specialists typically differentiate through niche capabilities such as biodegradable packaging expertise, bespoke sterile formats for surgical settings, or service models that bundle product supply with clinical training and protocol integration. Meanwhile, established manufacturers leverage distribution networks and product breadth to maintain relationships across retail, institutional, and military channels, putting pressure on newcomers to demonstrate clear, evidence‑based advantages. Mergers, licensing agreements, and contract manufacturing arrangements are common mechanisms to scale quickly or to access new chemistries and material technologies without duplicative capital outlay.
Across competitive profiles, commercial success increasingly depends on the ability to present compelling product dossiers that address safety, environmental impact, and clinical effectiveness. Companies that combine proven performance with transparent supply chains, robust compliance documentation, and adaptable packaging options are better positioned to win large procurement contracts and to expand into adjacent application segments such as veterinary care and sports team outfitting.
Practical and prioritized steps for manufacturers and buyers to enhance supply resilience, accelerate sustainable innovation, and win institutional and consumer channels
Industry leaders can translate current insights into concrete actions that enhance resilience, accelerate differentiation, and unlock new channels. First, prioritize supplier diversification and nearshore manufacturing options to reduce exposure to tariff volatility and to shorten lead times for critical inputs; this approach also supports faster response to regulatory shifts and localized product requirements. Second, invest in material and formulation innovation that targets reduced transport risks and improved biodegradability, thereby addressing both regulatory pressures and shifting consumer expectations for sustainable products. Third, develop modular product platforms that enable relatively rapid reconfiguration between disposable and reusable offerings, allowing tailored commercial propositions for consumer retail, clinical procurement, and athletic organizations.Additionally, firms should strengthen compliance and classification capabilities by embedding trade and regulatory expertise into product development cycles. Proactive engagement with regulatory authorities and participation in standards bodies can both smooth market entry and create early advantages in emerging regulatory regimes. From a commercial perspective, building evidence through clinical usability studies, patient comfort assessments, and durability testing for reusable formats will help secure institutional trust and long‑term contracts. Finally, adopt a channel segmentation approach that aligns packaging, labeling, and pricing with the distinct needs of consumer retail, ambulatory clinics, sports facilities, and veterinary practices to maximize adoption and reduce returns or dissatisfaction.
These pragmatic recommendations create a coherent action plan for leaders seeking to balance near‑term operational resilience with sustainable, innovation‑led growth.
A rigorous mixed‑methods research approach combining stakeholder interviews, technical literature review, and cross‑validated supply‑chain analysis to inform practical recommendations
The research underpinning these insights combined primary interviews, secondary literature synthesis, and cross‑validation via supply‑chain reconnaissance to ensure robust findings. Primary research included structured interviews with product development leads, procurement officers in clinical and sports settings, and logistics and regulatory specialists who manage chemical classification and transport. These conversations informed qualitative assessments of design priorities, sourcing constraints, and channel dynamics. Secondary research encompassed review of regulatory guidance documents, technical white papers on chemical formulations and packaging materials, and published protocols used by emergency services and clinical departments to contextualize application requirements.Data integrity was reinforced through triangulation: assertions derived from interviews were tested against technical literature and logistics data to ensure consistency, and divergent perspectives were explored further through targeted follow‑up interviews. The methodology also applied a scenario‑based approach to evaluate how variations in trade policy, raw material availability, and sustainability mandates could influence procurement and product development decisions. Wherever possible, empirical evidence from usage studies, clinical feedback, and material performance tests was used to ground recommendations in operational realities.
Finally, sensitivity to regulatory nuance and transport classification constraints guided the analytical framework, acknowledging that compliance pathways differ materially by region and by chemical composition. This mixed‑methods design produced a pragmatic synthesis intended to inform executive decision‑making without relying on proprietary market estimates or speculative forecasting.
Synthesis of the strategic levers - chemistry innovation, packaging sustainability, supply resilience, and regulatory engagement - that will determine long‑term competitive success
In conclusion, the instant cold pack sector stands at an inflection point where chemistry choices, packaging innovations, regulatory pressures, and trade dynamics converge to redefine competitive advantage. Manufacturers that proactively address safety and environmental concerns through alternative chemistries and biodegradable or fabric‑based packaging will be better poised to meet both institutional procurement standards and consumer sustainability expectations. At the same time, tariffs and trade complexity underscore the importance of supply‑chain diversification and near‑market manufacturing to preserve continuity of supply and control total landed costs.Application diversity-from sports medicine and injury management to clinical postoperative care and veterinary uses-creates multiple pathways for growth, but also requires targeted product configurations and evidence packages that resonate with specific end users. Competitive success will come to those organizations that can demonstrate performance, compliance, and lifecycle thinking while providing flexible commercial models that serve retail, institutional, and specialty channels. In short, aligning product innovation with regulatory foresight and procurement realities will be critical for converting technical advances into sustained commercial outcomes.
The synthesis presented here equips leaders with a clear view of strategic levers to prioritize: chemistry and material innovation, supply‑chain resilience, regulatory engagement, and channel segmentation. Executives and functional heads can use these levers to refine roadmaps, guide investments, and align organizational capabilities with the evolving needs of clinicians, consumers, and institutional buyers.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Disposable
- Reusable
- Chemical Composition
- Ammonium Nitrate-Based
- Calcium Ammonium Nitrate-Based
- Urea-Based
- Packaging Material
- Nylon
- Polyethylene
- Polypropylene
- Polyurethane
- Polyvinyl Chloride
- Cooling Duration
- 15-30 Minutes
- Less Than 15 Minutes
- More Than 30 Minutes
- Application
- Acute Injury
- Burns & Bruises
- Impact & Crush Injuries
- Sprains & Strains
- Chronic Pain
- Post-Procedural & Surgical Care
- Aesthetic Procedures
- Dental Surgery
- General Surgery
- Orthopedic surgery
- Acute Injury
- End User
- Hospitals & Clinics
- Ambulatory Care & Clinics
- Dental & Oral Clinics
- Emergency Medical Services (EMS)
- Physical Therapy & Rehab Centers
- Individuals / Households
- Military & Public Safety
- Sports & Fitness
- Gyms & Fitness Studios
- Outdoor & Adventure Sports
- Veterinary & Animal Care
- Hospitals & Clinics
- Distribution Channel
- Offline
- Retail Pharmacies & Drug Stores
- Supermarkets & Hypermarkets
- Online
- Brand Websites
- eCommerce Platforms
- Offline
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Cardinal Health, Inc.
- 3M Company
- Acme United Corporation
- Boen Healthcare Co., Ltd.
- Carex Health Brands
- Chongqing New World Trading Co., Ltd.
- Coldstar International Corporation
- Collateral Medical Pvt Ltd, Inc.
- Cryopak Industries Inc.
- D S Enterprises
- Dispotech srl
- Dukal Corporation
- Dynarex Corporation
- EverReady First Aid, LLC
- First Aid Only, Inc. by ACME UNITED CORPORATION
- Gel Frost Packs Kalyani Enterprises
- GVS International
- HART Health, Inc.
- Jiangsu Yishun Medical Equipment Co., Ltd.
- Kobayashi Pharmaceutical Co., Ltd.
- McKesson Medical-Surgical Inc.
- Medline Industries, LP
- Mueller Sports Medicine, Inc.
- O&M Halyard, Inc.
- Performance Health LLC
- Senso Medi Systems
- Shrushi Polymers Private Limited.
- Suzhou Sunmed Co., Ltd. A
- TAN90 Thermal Solutions Private Limited.
- Vidhya Enterprises
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Instant Cold Pack market report include:- Cardinal Health, Inc.
- 3M Company
- Acme United Corporation
- Boen Healthcare Co., Ltd.
- Carex Health Brands
- Chongqing New World Trading Co., Ltd.
- Coldstar International Corporation
- Collateral Medical Pvt Ltd, Inc.
- Cryopak Industries Inc.
- D S Enterprises
- Dispotech srl
- Dukal Corporation
- Dynarex Corporation
- EverReady First Aid, LLC
- First Aid Only, Inc. by ACME UNITED CORPORATION
- Gel Frost Packs Kalyani Enterprises
- GVS International
- HART Health, Inc.
- Jiangsu Yishun Medical Equipment Co., Ltd.
- Kobayashi Pharmaceutical Co., Ltd.
- McKesson Medical-Surgical Inc.
- Medline Industries, LP
- Mueller Sports Medicine, Inc.
- O&M Halyard, Inc.
- Performance Health LLC
- Senso Medi Systems
- Shrushi Polymers Private Limited.
- Suzhou Sunmed Co., Ltd. A
- TAN90 Thermal Solutions Private Limited.
- Vidhya Enterprises
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
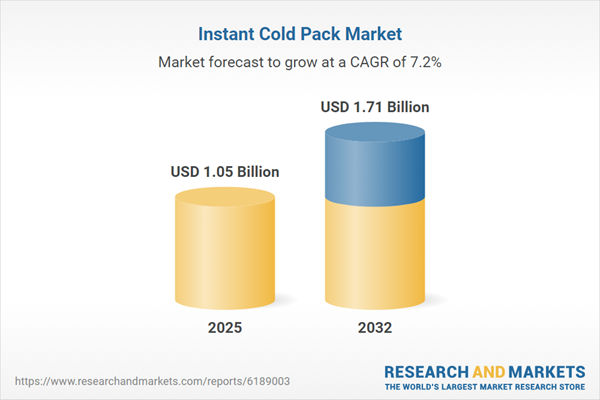
| Estimated Market Value ( USD | $ 1.05 Billion |
| Forecasted Market Value ( USD | $ 1.71 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |